
(a)
Interpretation:
Anticipating the isolation of the potentially aromatic hydrocarbon B, a group of chemists irradiated compound A with ultraviolet light. Compound C was obtained as a product instead of B.
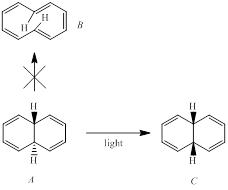
The reason for compound B might be expected to be unstable in spite of its cyclic array of 4n+2 π electrons is to be stated.
Concept introduction:
The stability of
(b)
Interpretation:
The formation of compound B is allowed by the pericyclic selection rules is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The word ‘pericyclic’ means around the cycle. Notably, the pericyclic reactions are classified as electrocyclic, cycloaddition and sigmatropic reactions based on the activation of reaction, the number of electrons involved and the stereochemistry outcome of the reactions. The selection rules of pericyclic reaction deal mostly on the
(c)
Interpretation:
The formation of the observed product C is to be explained.
Concept introduction:
Pericyclic reactions deal with electrons shift in a concerted manner. Moreover, in these reactions the breaking of reactant bonds and the formation of product bonds are formed at the same time.i.e., without the formation of intermediates. The selection rules of pericyclic reaction deal mostly on the rates of reaction and does not tell anything about position of equilibrium and equilibrium constant values.
Trending nowThis is a popular solution!

Chapter 28 Solutions
Organic Chemistry
- (a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forwardRank the compounds in each group in order of increasing reactivity in electrophilic aromatic substitution: (a) C6H6, C6H5Cl, C6H5CHO, C6H5OCH3; (b) C6H5CH3, C6H5NH2, C6H5CH2NH2, C6H5CONH2.arrow_forwardCompound A has molecular formula C5H8Br4 but shows only one singlet in the 1H-NMR spectrum. Suggest a structure for A and explain your reasoning.arrow_forward
- Methotrexate, a drug that inhibits the metabolism of folic acid, is used in the treatment of a variety of cancers and autoimmune disorders such as rheumatoid arthritis. (a) GIve the hybridization of each N atom in methotrexate. (b) In what type of orbital does the lone pair of each N reside? (c) Explain why the bicyclic ring system that contains four N atoms is aromatic.arrow_forwardThe treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formula C5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forward(a) The 'H-NMR spectrum of cyclobutanone shows two signals - signal A at 3.00 ppm and signal B at 1.95 ppm. Give the multiplicity of each signal. cyclobutanone (b) When cyclobutanone is treated with D20 and NaOD, the only signal observable in the 1H-NMR is a singlet at 2.00 ppm. Explain why this is the case. [Note: Deuterium atoms do not display signals in the TH-NMR spectrum]arrow_forward
- The treatment of (CH3)2C = CHCH2Br with H2O forms B (molecular formula C5H10O) as one of the products. Determine the structure of B from its H NMR and IR spectra.arrow_forwardThe bicyclic heterocycles quinoline and indole undergo electrophilic aromatic substitution to give the products shown. (a) Explain why electrophilic substitution occurs on the ring without the N atom for quinoline, but occurs on the ring with the N atom in indole. (b) Explain why electrophilic substitution occurs more readily at C8 than C7 in quinoline. (c) Explain whyelectrophilic substitution occurs more readily at C3 rather than C2 of indole.arrow_forwardThe bicyclic heterocycles quinoline and indole undergo electrophilic aromatic substitution to give the products shown. (a) Explain why electrophilic substitution occurs on the ring without the N atom for quinoline, but occurs on the ring with the N atom in indole. (b) Explain why electrophilic substitution occurs more readily at C8 than C7 in quinoline. (c) Explain why electrophilic substitution occurs more readily at C3 rather than C2 of indole.arrow_forward
- Compound P has molecular formula C5H9C102. Deduce the structure of P from its 1H and 13C NMR spectra.arrow_forwardThe endiandric acids comprise a group of unsaturated carboxylic acids isolated from a tree that grows in the rainforests of eastern Australia. The methyl esters of endiandric acids D and E have been prepared from polyene Y by a series of two successive electrocyclic reactions: thermal ring closure of the conjugated tetraene followed by ring closure of the resulting conjugated triene. (a) Draw the structures (including stereochemistry) of the methyl esters of endiandric acids D and E. (b) The methyl ester of endiandric acid E undergoes an intramolecular [4 + 2] cycloaddition to form the methyl ester of endiandric acid A. Propose a possible structure for endiandric acid A.arrow_forwardA) Provide the reagent and reaction mechanism to show how the reactants and products in the following reaction can interconvert B) under what conditions would the reaction I) favour reactants, II) favour the products and III) why?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





