
Interpretation:
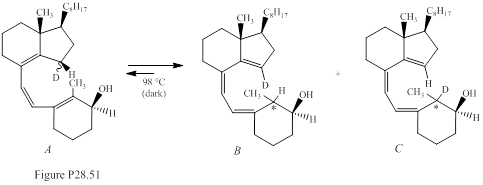
In 1985, two researchers at the University of California, Riverside, carried out the reaction given in Fig. P28.51.The equilibrium mixture contained compound A (22%), a single stereoisomer of B (47%), and a single stereoisomer of C (31%).The stereochemistry of compounds B and C at the carbon marked with the asterisk (*) is to be predicted.
Concept introduction:
Sigmatropic reaction can be described as the migration of allylic sigma bond at one end of the π-electron system to the other end of the π-electron system as an uncatalyzed intramolecular reaction. The formation of sigma bond at 3, 3-position of a 1, 5-diene is called as cope rearrangement. Notably, [3, 3] sigmatropic reaction of allyl vinyl ether is termed as Claisen rearrangement.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- 4) What happens to the stereochemistry during and SN2 reaction? Why? Provide a reaction to illustrate this.arrow_forwardDraw the structure of the expected organic produect formed in the following reactions including correct relative stereochemistry; if the reaction is racemic indicate this by either drawing both enantiomers or drawing one and writing racemic. Assume all reagent are present in excess unless otherwise noted. If no reaction occurs, state "No Reaction".arrow_forwardObserve and identify what's the general reaction scheme and explain it.arrow_forward
- (a) Recall, from WP 14.26 (and from lecture), cyclopentadienone and cycloheptatrienone differ drastically in reactivity and stability. One is very stable and unreactive, and the other one is very reactive and rapidly undergoes a dimerization reaction. Which one is particularly stable? Explain why. For the one which is extremely reactive, explain why, but also draw the structure of the dimer which is formed rapidly.arrow_forwardPyrethrin II and pyrethrosin are two natural products isolated from plants of the chrysanthemum family. Pyrethrin II is a natural insecticide and is marketed as such. Q, Calculate the index of hydrogen deficiency for each of these natural productsarrow_forwardFor each pair of compounds, a state which compound is the better SN2 substrate. (a) 2-bromobutane or isopropyl bromidearrow_forward
- Draw the structure of the expected organic produect formed in the following reactions including correct relative stereochemistry; if the reaction is racemic indicate this by either drawing both isomers or drawing one and writing racemic. Assume all reagent are present in excess unless otherwise noted. If no reaction occurs, state "No Reaction".arrow_forward(i) Explain with the aid of a reaction mechanism why the synthesis is highly diastereoselective. (ii) Draw the structures of the enantiomer, and one epimer of J, and give definitions of these terms.arrow_forwardConsider the tetracyclic compound with rings labeled A–D. (a) Which ring is the most reactive in electrophilic aromatic substitution? (b) Which ring is the least reactive in electrophilic aromatic substitution?arrow_forward
- For the following, complete the reactions with the predominant product or products. You must indicate stereochemistry with wedges and dashes. If a racemic mixture is created, you must write "racemic" under the structures.arrow_forwardstion 9 of 14 Alkenes can be converted to alcohols by hydroboration-oxidation. (a) Draw the structure of the alcohol or alcohols formed in the reaction sequence. Clearly indicate stereochemistry by drawing a wedged bond, a dashed bond and two in-plane bonds chiral carbon. Draw hydrogen atoms that are connected to wedge-and-dash bonds.(b) Characterize the product or products of per each the reactions. Be sure to draw hydrogens on oxygen, where applicable. Select Draw Rings More Erase / /の|/| 1. B2H6, diglyme (a) 2. H2O2, HO¯, H2Oarrow_forwardThe saccharide shown here is present in some plant-derived foods. (a) Indicate the anomeric carbon atom(s) in this structure by drawing a circle around the atom(s) or by drawing an arrow pointing to the atom(s). (b) Would this saccharide give a positive result with Benedict’s reagent? Why or why not? (c) Would this saccharide give a positive result with Barfoed’s reagent? Why or why not? (d) Would this saccharide give a positive result with Seliwanoff’s reagent? Why or why not? (e) In a separate set of experiments, the saccharide solution was treated with a reagent that breaks glycosidic bonds. After this treatment, would any of the three assays give different results? Be sure to indicate which assay results would be different and give a reason.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


