
Concept explainers
(a)
Interpretation:
The stereochemistry of compounds B and C in Fig. P28.31 is to be predicted.
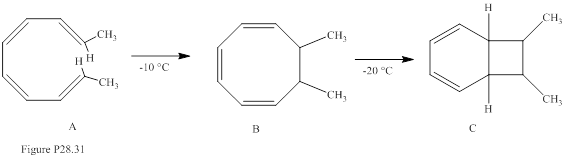
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner. Selection rules of electrocyclic reactions are;
| No. of electrons | Activation mode | Stereochemistry of rotation |
| 4n | Thermal Photochemical |
Con Dis |
| 4n + 2 | Thermal Photochemical |
Dis Con |
(b)
Interpretation:
The stereoisomer of A also gives compound C on heating is to be stated.
Concept introduction:
When the diene is having the oxygen at C3 or C4 position the sigmatropic reaction is called as oxy cope reaction. Notably, when the alkoxide fragment is involved it is referred as anionic oxy cope reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- Account for the following:(i) Aniline does not give Friedel-Crafts reaction.(ii) Ethylamine is soluble in water whereas aniline is not.(iii) pKb of methylamine is less than that of aniline.arrow_forward(b) Distinguish each as aromatic or antiaromatic in terms of electronic basis. Explain which one is more stable. (i) Compound A Compound B (ii) Compound C Compound D (iii) Compound E Compound Farrow_forwardDeduce the structures of compound A,B,C and Darrow_forward
- Provide the reagents and solvents (where appropriate) needed to bring about the following transformations. (a) CI (b)arrow_forwardCompound F may be synthesised by the method attached Draw the isomer of compound B and explain which one would be the major product and why.arrow_forwardHow would you account for the following :(a) Electrophilic susbstitution in case of aromatic amines takes place more readily than benzene.(b) Ethanamide is a weaker base than ethanamine.arrow_forward
- (a) Classify the enolates to 'kinetic' and 'thermodynamic.' (b) Compare the stabilities of two enolates. (c) Describe the three reaction conditions of 'thermodynamic enolates' and explain why.arrow_forward(a) Discuss the aromaticity of a six membered heterocyclic compound which is weak base in nature. (b) Discuss about the stability factors of the reaction intermediates, which involved in a name reaction "Wittig rearrangement".arrow_forwardDeduce the structure of compound C.arrow_forward
- Give IUPAC names for the following structures. (If appropriate, specify relative stereochemistry.) (a) (b) S Sarrow_forward(a) Why are alkyl halides insoluble in water? (b) Why is Butan-l-ol optically inactive but Butan-2-ol is optically active? (c) Although chlorine is an electron withdrawing group, yet it is ortho-, Para- directing in electrophilic aromatic substitution reaction. Why?arrow_forward. (a) 2,5-Dimethyl-1,1- cyclopentanedicarboxylic acid (I) may be prepared as two optically inactive substances (A and B) of different m.p. Draw their possible structures. (b) Upon heating, A yields two 2,5- dimethylcyclopentanecarboxylic acid structures (II), and B yields only one. Assign structures to A and B. HO2C Co 2H HO 2C, Harrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





