
Interpretation:
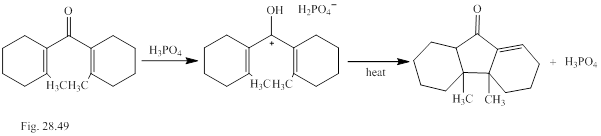
Ions as well as neutral molecules undergo pericyclic reactions. Classify the pericyclic reactions of the cation involved in the transformation shown in Fig. P28.49.The methyl groups are cis or trans is to be explained.
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner. In phase orbital overlap results in symmetry allowed electrocyclic reactions. Selection rules of electrocyclic reactions are;
| No. of electrons | Activation mode | Stereochemistry of rotation |
| 4n | Thermal Photochemical |
Con Dis |
| 4n + 2 | Thermal Photochemical |
Dis Con |
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- (a) Which is the correct structure of the alkyl halide substrate? (b) Which is the correct structure of the nucleophile?arrow_forwardProvide the curved-arrow mechanism to account for the following electrophilic aromatic substitution reaction. .CI Cl2 FeCl3arrow_forward(a) Why are alkyl halides insoluble in water? (b) Why is Butan-l-ol optically inactive but Butan-2-ol is optically active? (c) Although chlorine is an electron withdrawing group, yet it is ortho-, Para- directing in electrophilic aromatic substitution reaction. Why?arrow_forward
- 4) What happens to the stereochemistry during and SN2 reaction? Why? Provide a reaction to illustrate this.arrow_forwardSuggest a reason why wittig reactions usually give mixtures of cis and trans isomers. which of the cis or trans isomer is the. major product? explain.arrow_forwardtwo positions of anthracene sometimes react more like polyenes thanlike aromatic compounds.(a) Draw a Kekulé structure that shows how the reactive positions of anthracene are the ends ofa diene, appropriate for a Diels–Alder reaction.(b) The Diels–Alder reaction of anthracene with maleic anhydride is a common organic labexperiment. Predict the product of this Diels–Alder reaction.arrow_forward
- 22:43 10. Provide the following: the resonance structure of the intermediate that can resonate into the other intermediate that is given the product that results from the given resonance structure Specify which product is the kinetic product and which is the thermodynamic product . OH 50% H₂SO4 H~arrow_forwardExplain with the help of electronic effects, ‘generally haloalkenes undergo nucleophilic substitution reactions whereas haloarenes undergo electrophilic substitution reactions’.arrow_forwardAn organic compound A of unknown structure was found to have a molecular formula C8H16. When A was poured in water and heated, compound B having a molecular formula C8H18O was formed. B upon heating with sulfuric acid was converted to C as the major product which is identical to A. Ozonolysis of C gave one molecule each of two different products D and E, both having a molecular formula C4H8O. Write the reactions involved and determine the structure of A,B,C,D and E.arrow_forward
- (a) Draw vinyl halide and aryl halides structure. By using your drawing structure either the given structure is reactive or unreactive halides in Friedel-Crafts alkylation.arrow_forwardIn the following molecules, the carbon with the double bond to oxygen is called the carbonyl carbon. Which molecule has the more electrophilic carbonyl carbon? Explain your reasoning. 11) For each of the following molecules, predict two possible products that can be formed by reaction with sodium cyanide (NaCN), then rank the two products in terms of stability. b) O a) ugatearrow_forward11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forward
