
Concept explainers
Interpretation:
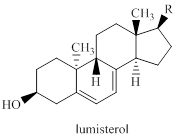
When previtamin D2 (which is identical to previtamin D3, p. 1478, except for the R-group) is isolated and irradiated, ergosterol is obtained along with a stereoisomer, lumisterol. The origin of lumisterol is to be explained mechanistically.
Concept introduction:
Electrocyclic reactions are a pericyclic reaction which occur intramolecularly. These reactions will result in the formation of ring compounds under the influence of heat or light. Notably, in this process one new sigma bond is formed and one old π-bond is consumed. Intriguingly, the reverse ring opening electrocyclic reaction can also be possible to occur under the same reaction mechanism but in reverse manner. In phase orbital overlap results in symmetry allowed electrocyclic reactions. Selection rules of electrocyclic reactions are;
| No. of electrons | Activation mode | Stereochemistry of rotation |
| 4n | Thermal Photochemical |
Con Dis |
| 4n + 2 | Thermal Photochemical |
Dis Con |
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry
- Identify compounds A, B, and C. a.) Compound A has molecular formula C8H12 and reacts with two equivalents of H2. A gives HCOCH2CH2CHO as the only product ofoxidative cleavage with O3 followed by CH3SCH3. b.) Compound B has molecular formula C6H10 and gives(CH3)2CHCH2CH2CH3 when treated with excess H2 in the presence ofPd. B reacts with NaNH2 and CH3I to form compound C (molecularformula C7H12).arrow_forwardAmmonia and amines react with epoxides with the same stereospecificity as anionic nucleophiles. Draw a sawhorse or Newman projection formula for the product of the reaction shown, clearly showing the stereochemistry at both chirality centers. What are the Cahn–Ingold–Prelog R,Sdescriptors for these chirality centers in the reactant and the product?arrow_forwardGamma(y)-amino butyric acid (GABA) is a neurotransmitter (a chemical that is used to send signals from one neuron to another) of the mammalian central nervous system. In order to understand how GABA works, conformationally restricted analogues, such as compound 1, have been made. During a synthesis of compound 1, compound 2 was subjected to allylic bromination using NBS and a radical initiator (AIBN) instead of light (Aust. J. Chem. 1981, 34, 2231-2236). H₂N. CH3 GABA CO₂H CH3 dddd CH3 H₂N Modify the structures given below to draw all eight possible allylic bromides that can be formed when compound 2 undergoes allylic bromination, considering all possible regiochemical and stereochemical outcomes. You can use the single bond tool to add/remove pi bonds. CH3 CO₂H CH3 solddd CH3 CH3 2 CH₂ CO₂Etarrow_forward
- Quinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed to synthesize the drug?arrow_forwardSaquinavir (trade name Incirase) belongs to a class of drugs called protease inhibitors, which are used to treat HIV. Locate all the stereogenic centers in the drug saquinavir. H. H. N. N. H. NH2 OH O: saquinavir Trade name: Invirase NH O:arrow_forwardBoth pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, where as pyrrole is unreactive. What is the best explanation for this observed reactivity.arrow_forward
- How would we know using aromatic vicinal coupling that the c-c bond was formed between compound B and C to for the product A? (HO)₂B. Br ------ compound A compound B compound Carrow_forward(a) Explain why an alkylamine is more basic than ammonia?(b) How would you convert(i) Aniline to nitrobenzene (ii) Aniline to iodobenzenearrow_forwardPractice Problem 19.54 Z Your answer is partially correct. Try again. Predict the major product(s) (A - K) from the treatment of acetone with the following compounds (a-c): NH2 HO Eto OEt A: B: C: D: E: F: OH OH но CN G: H: I: J: (a) [H*], excess EtOH, (-H20) Major Product(s): (ь) NaBH4, Meон B Major Product(s): (c) LAH followed by H20 Major Product(s): SHOW HINTarrow_forward
- Rhodamine B is useful dye prepared in a manner very similar to fluorescein. Due to the nitrogen donating groups, rhodamine B absorbs 550 nm light and fluoresces 580 nm light. a) What color would you expect for a rhodamine B solution? b) What is the color of the fluoresced (emitted light)?arrow_forwardThe saccharide shown here is present in some plant-derived foods. (a) Indicate the anomeric carbon atom(s) in this structure by drawing a circle around the atom(s) or by drawing an arrow pointing to the atom(s). (b) Would this saccharide give a positive result with Benedict’s reagent? Why or why not? (c) Would this saccharide give a positive result with Barfoed’s reagent? Why or why not? (d) Would this saccharide give a positive result with Seliwanoff’s reagent? Why or why not? (e) In a separate set of experiments, the saccharide solution was treated with a reagent that breaks glycosidic bonds. After this treatment, would any of the three assays give different results? Be sure to indicate which assay results would be different and give a reason.arrow_forward(a) What product is formed by the Claisen rearrangement of compound Z? (b) Using what you have learned about ring-closing metathesis in Chapter 26, draw the product formed when the product in part (a) is treated with Grubbs catalyst. These two reactions are key steps in the synthesis of garsubellin A, a biologically active natural product that stimulates the synthesis of the neurotransmitter acetylcholine. Compounds of this sort may prove to be useful drugs for the treatment of neurodegenerative diseases such as Alzheimer’s disease.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
