
Concept explainers
(a)
Interpretation:
Chiral carbon atom in naproxen and L-DOPA has to be identified.
Concept Introduction:
Chirality: It refers to an atom in a molecule that contains four different substituents.
(b)
Interpretation:
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which are determine the characteristic reactions taking place in it.
Alcohol: It is an organic compound where it contains at least one
Carboxylic acid: One
Ether: Ether is a group of organic compound where two aryl or alkyl groups are connected by an oxygen atom. It is represented as
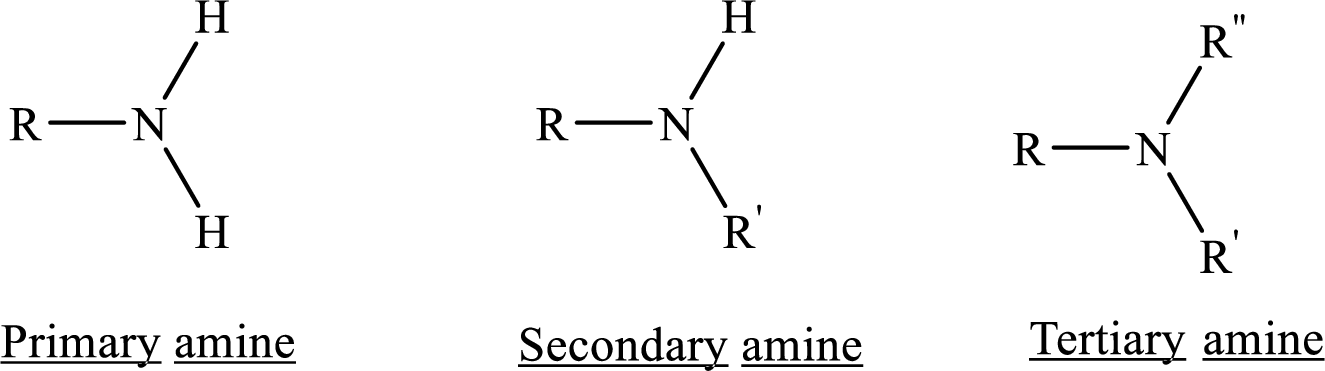
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
Aromatic Compounds: Compounds that are planar, cyclic and having
(c)
Interpretation:
Structural formula for the mirror images of naproxen and L-DOPA has to be identified.
Concept Introduction:
In chemistry, structure is the arrangement of
In structural formula,
- All of the atoms are shown at each end and intersection of bonds
- H atoms are shown
Enantiomers: The presence of atom with non-super impossible mirror image is defined as enantiomers which are given
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- 2. Soap can be made using a variety of different fats, oils and bases. Suppose a student wanted to make a soft, liquid soap that lathers easily. What type(s) of fat/oil and base would you recommend? How would this differ from making a solid bar of soap? 3. Vegetable oils are typically liquid at room temperature, yet Crisco (which is made of vegetable oil) is solid at room temperature. Explain why/how this is the case. 4. Explain, with the use of a diagram how soap molecules work to clean “dirt” particles. 5. Amylase is a natural enzyme found in saliva. Describe this enzyme’s function. 6. Lactose is a sugar found in milk and other dairy products. Explain how it is broken down in most individuals. Explain lactose “intolerance”.arrow_forward1. explain the meaning of the terms bioplastic and biodegradable. 2. describe the properties of the two films that you have made. 3.why hydrochloric acid is use to make potato plastic film? 4. what difference did the addition of propane-1,2,3-triol make? 5.why does adding the propane-1,2,3-triol change the properties of the plastic?arrow_forward1. The Ability of a substance to exist in different crystalline form a. Lattice b. Polymorphism c. Crystallization d. Amphoterism 2. A drug can exert its pharmacological effect only if it is a. Protein bound b. Protein unbound c. Free drug d. Both B & C e. Both A & C 3. In order for the drug to be ready and available for absorption, it must be release first from its dosage form with the exception of: a. Capsule b. Tablet c. Solution d. Suspension 4. All of the following are true, except a. Solubility increase with decrease particle size b. Solubility increase with increase surface area c. Solubility increase with increase particle size d. Solubility decrease with decrease surface area 5. The rate in which the drug appears in the bloodstream is also known as a. Half-life b. Potency c. Bioavailability d. Area under the curvearrow_forward
- the analysis of a mixture of hydrocarbon cracking products, all of which are able to be distilled, but distillation is unable to separate them cleanly. A. a pharmaceutical product containing 2 stereoisomers of the active ingredient, both of which are fairly polar, aromatic, water soluble, and decompose rather than boil. B. C. The separation of a mixture of water-soluble globular proteins of various sizes. The analysis of a mixture of reactor gases that includes methane, hydrogen, carbon dioxide, carbon monoxide, and nitrogen. D.arrow_forward4. List the number for each molecule in the order they would be separated from the bottom of a fractional distillation tower to the top. Use the following information to answer the next question. Alkanes 1. 2-methyloctane 2. CH (g) 3. CH 4. CH₂(g) 3. Draw and name two line structural diagrams that have the structural formula C8H14. Identify if your molecule is aliphatic or aromatic and if it is either saturated or unsaturated.arrow_forward1. What functional group increases the boiling point of straight chain primary alcohols and carboxylic acids by 18 degrees Celsius? methane methylane ethylene methylene 2. What type of bonds are present between alcohol molecules that allow them to have a higher boiling point than saturated hydrocarbons? A. Van der Waals forces B. hydrogen bond C. london forces D. dipole-dipole bonds 3. It is a compound composed of 2 similar subunits or held together by ionic or covalent bonds. primer chiral monomer dimerarrow_forward
- Indicate whether or not each of the following statements is TRUE or FALSE. Justify your answer with ONE or TWO sentences. i. Lipids are considered to be more soluble in water than in other solvents. ii. The many -OH groups present in carbohydrates such as glucose make them quite insoluble in water and blood. ii. Carbohydrate structure shows that water molecules are connected to carbon atoms. iv. Hydrocarbons contain carbon, hydrogen and oxygen. A monosaccharide can be hydrolyzed to smaller units. V.arrow_forward2. Why is it important to know the properties of common organic liquid materials? To know A. the uses of the liquids B. how these liquids affect people C. possible dangers from these kinds of materials D. all of the above 3. Why are carbon atoms able to form many organic compounds? Carbon atoms A. attract electrons from other atoms. B. have strong attraction to other elements C. can form many types of bonds with other carbon D. none of the above 4. Which alkene will most likely have the highest boiling point? B. hexene A. ethene C. pentene D. propene 5. Which are TRUE about organic compounds? They A. contain calcium B. contain carbon C. can be produced artificially D. can be produced by living organismsarrow_forwardIndicate whether or not each of the following statements is TRUE or FALSE. Justify your answer with ONE or TWO sentences. i. Lipids are considered to be more soluble in water than in other solvents. ii. The many -OH groups present in carbohydrates such as glucose make them quite insoluble in water and blood. iii. Carbohydrate structure shows that water molecules are connected to carbon atoms. iv. Hydrocarbons contain carbon, hydrogen and oxygen. v. A monosaccharide can be hydrolyzed to smaller units.arrow_forward
- 3. Draw the structures for the primary artificial sweetening agents present in SplendaⓇ and Equal®. What are the names of these compounds? 4. Draw the structure of sucrose (table sugar). How do the structures of artificial sweetening agents shown above relate to the structure of sucrose? 5. Draw the reaction that will be performed in today's experiment.arrow_forwardWhat method did the Persian healer and alchemist, Avicenna, develop that led to the art of aromatherapy? Choose one answer. a. identification of essential oils b. tainting of essential oils c. blending of essential oils d. distillation of essential oilsarrow_forward1. Draw the structures of the first two synthetic sweeteners and indicate when they were discovered. 2. List two reasons why artificial sweetening agents are important to the food industry. 3. Draw the structures for the primary artificial sweetening agents present in SplendaⓇ and Equal®. What are the names of these compounds? 4. Draw the structure of sucrose (table sugar). How do the structures of artificial sweetening agents shown above relate to the structure of sucrose? 5. Draw the reaction that will be performed in today's experiment.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





