
Concept explainers
Interpretation:
The range of chemical shifts in the given compound is to be determined.
Concept introduction:
Nuclear magnetic resonance (NMR) is one of the most capable analytical techniques used for determining the
A compound containing protons or carbon-13 when placed under very strong magnetic field and treated with
Nuclear magnetic resonance spectroscopy is a graph showing characteristic energy absorption frequencies and intensities of a compound under magnetic field.
Chemical shifts are positions of signals along the x-axis in the nuclear magnetic spectroscopy. It gives an idea on how many different hydrogen atoms are present in a given H-NMR for a particular compound.
More electronegative atoms attached to the group, more the protons are deshielded; more will be the chemical shift value.
Answer to Problem 1PP
Solution:
Chemical shifts for ethyl acetate are at
Explanation of Solution
Structure of ethyl acetate
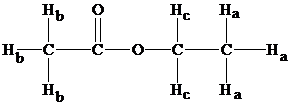
From the structure, we have three types of hydrogens and from table 9.1 chemical shifts result in the values as follows:
The chemical shifts are in the ranges of
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry
- The following 1H NMR peaks were recorded on a spectrometer operating at 200 MHz. Convert each into δ units. (a) CHCl3; 1454 Hz (b) CH3Cl; 610 Hz (c) CH3OH; 693 Hz (d) CH2Cl2; 1060 Hzarrow_forwardOrganic Chemistry - How many signals would you expect in the 1H NMR spectrum of HOCH2CH2CH2CH2OH?arrow_forwardShown below is the Proton NMR of a hydrocarbon of the formula C11H16. Given the number of hydrogen atoms represented by each of the 3 integrals, what is the structure of the compound?arrow_forward
- How many peaks would the 1H NMR spectrum of the following molecule have?arrow_forwardWhat is the molecular formula structure and its proton environment of these two spectrum?arrow_forwardPredict the approximate chemical shifts of the protons in the following compounds. (a) benzene (b) cyclohexanearrow_forward
- Use the following information to propose a molecular formula for nootkatone, a compound partly responsible for the characteristic odor of grapefruit. Nootkatone contains the elements C, H, and O, has five degrees of unsaturation, and a molecular ion in its mass spectrum at m/z = 218.arrow_forwardUse the following information to propose a molecular formula for nootkatone, a compound partly responsible for the characteristic odor of grapefruit. Nootkatone contains the elements C, H, and O, has ve degrees of unsaturation, and a molecular ion in its mass spectrum at m/z = 218.arrow_forwardDraw the structural formulas of the following compounds and indicate the number of NMR signals that would be expected for each compound. (a) methyl iodide (b) 2,4-dimethylpentane (c) cyclopentane (d) propylene (propene)arrow_forward
- What is the 1H NMR data (chemical shift, integration, multiplicity (specify as singlet, doublet, triplet,etc.) of the following two compounds?arrow_forwardConsider the aromatic compound 4-isopropyl-benzonitrile. (Benzonitrile is a benzene ring with the nitrile group on position 1.) How many signals for non-equivalent types of protons will be in its proton NMR spectrum?arrow_forwardThe 1H-NMR spectrum of the compound with the closed formula C8H16O2 is given below. Find the possible structure of the compound by analyzing the spectrum.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

