
(a)
Interpretation: The potential organic product that could result from the below reaction should be identified.
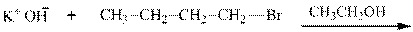
Concept introduction: Bimolecular substitution or
A general
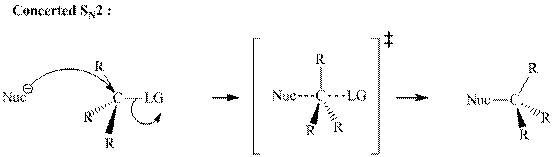
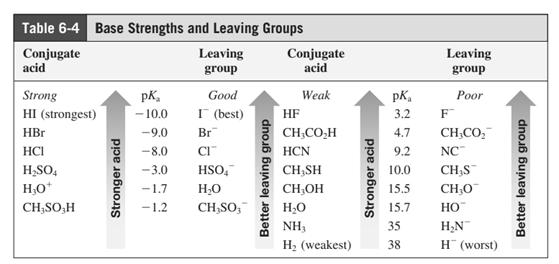
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of the strength of bases is as follows:
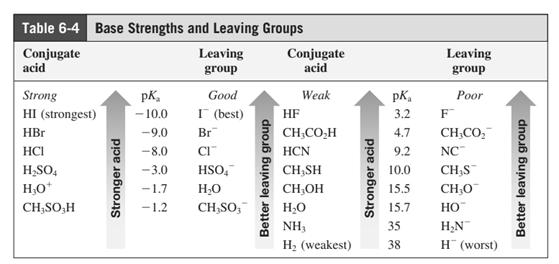
(b)
Interpretation:The potential organic product that could result from the below reaction should be identified.
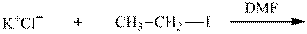
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of the strength of bases is as follows:
(c)
Interpretation:The potential organic product that could result from the below reaction should be identified.
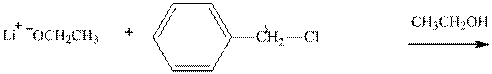
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(d)
Interpretation: The potential organic product that could resultfrom the belowreaction should be identified and no reaction should be indicated if no product is possible.
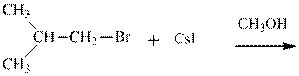
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(e)
Interpretation: The potential organic product that could result from the belowreaction should be identified and no reaction should be indicated if no product is possible.
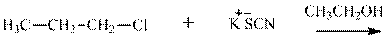
Concept introduction: Bimolecular substitution or
(f)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
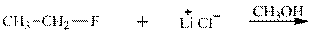
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(g)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
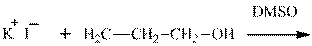
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(h)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
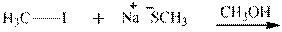
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(i)
Interpretation: The potential organic product that could result from below reaction should be identified and no reaction should be indicated if no product is possible.
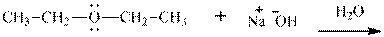
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(j)
Interpretation: The potential organic product that could result from below reaction should be identified and no reaction should be indicated if no product is possible.
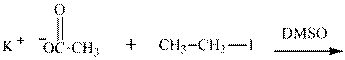
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- Alcohols are very useful starting materials for the production of many different compounds. The following conversions, starting with 1-butanol, can be carried out in two or more steps. Show the steps (reactants/catalysts) you would follow to carry out the conversions, drawing the formula for the organic product in each step. For each step, a major product must be produced. (See Exercise 62.) (Hint: In the presence of H+, an alcohol is converted into an alkene and water. This is the exact reverse of the reaction of adding water to an alkene to form an alcohol.) a. 1-butanol butane b. 1-butanol 2-butanonearrow_forwardName the type of reaction SO3 S OH H,SO 4. O Sulfonation O Carboxylation O Halogenation O Hydrohalogenationarrow_forwardConsider the following chemical reaction. HNO3, H2SO4 Predict the product of this reaction. Select the name that best corresponds to the product. O 2,4,6-trinitrotoluene O sulfuric acid O benzene O nitrobenzenearrow_forward
- Refer to the following structures: K (V) -OH O I, II, III OI, VI O III, V OI, IV OVI DA NH₂ ✓ CH₂-CH-C²-OH Which of these structures are esters? ورے • CH₂ TD ~^^N-CH₂arrow_forwardDehydration of 2-methyl-2-pentanol forms one major and one minor organic product. Draw the structures of the two organic products of this reaction. : OH H2SO4 Major product + Minor productarrow_forwardThe acid-catalyzed dehydration of 2,3-dimethyl-3-pentanol yields three alkene products. What are the names of the three alkenes? Which of the three alkenes is the major product?arrow_forward
- What is the slow, rate-determining step, in the acid-catalyzed dehydration of 2- butanol? Loss of a b-hydrogen from the carbocation to form an alkene. Protonation of the alcohol to form an oxonium ion. Loss of water from the oxonium ion to form a carbocation. The simultaneous loss of a B-hydrogen and water from the oxonium ion.arrow_forwardWhich alcohols can be prepared as a single product by hydroboration–oxidation of an alkene? Which alcohols can be prepared as a singleproduct by the acid-catalyzed addition of H2O to an alkene?arrow_forwardDraw the structures of two alkenes that would react to form the alcohol below by acid-catalyzed hydration. Your alkenes should be different regioisomers that yield the alcohol as the major product without requiring rearrangement. Click and drag to start drawing a structure. Ö + T x H₂SO4 H₂0 OH 8 昆山网图] E₂ 00. Ararrow_forward
- Acid-catalyzed dehydration of secondary and tertiary alcohols proceeds through an E1 mechanism. The first step is the protonation of the alcohol oxygen to form an oxonium ion. Dehydration of 3-methyl-2-butanol forms one major and two minor organic products. Draw the structures, including hydrogen atoms, of the three organic products of this reaction. н н :бн н Нас. Нас. CHз CH3 ČH3 CH3 3-methyl-2-butanol an oxonium ion Major Product Minor Product Minor Productarrow_forwardThe following chemical reaction is used to synthesize a flavouring agent that has an aroma similar to bananas. H₂SO4(aq) CH3COOH(1) + CH₂(CH₂)₂OH(1) I || Identify the type of reaction that is represented by this synthesis. Select one: O hydrogenation O esterification O addition O substitution O elimination Identify the functional group and the IUPAC name for each of the three compounds in the reaction below: H₂SO4(aq) I Compound Functional Group IUPAC Name II CH₂COOH(1) + CH₂(CH₂)₂OH(1) I || III CH3COO(CH₂)₂CH₂(1) + H₂O(1) III IV ◆ → ◆ CH3COO(CH₂)₂CH₂(1) + H₂O(1) IV ||| ◆ ◆arrow_forward4. Give the major organic product(s) for each of the following reactions. CH₂OH Nal Br CH3 CH₂Sarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




