
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 22.75SP
The Knoevenagel condensation is a special case of the aldol condensation in which an active methylene compound reacts with an
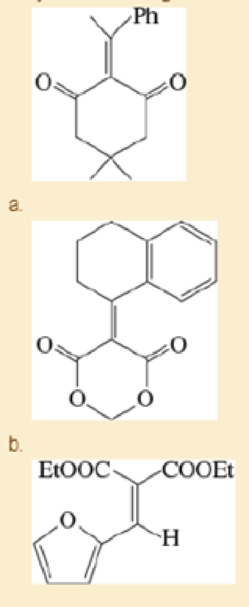
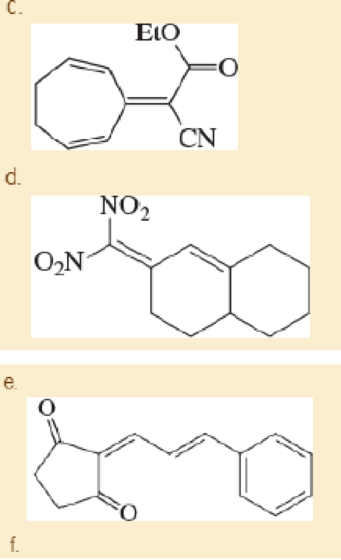
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2
H3C
H3C
H
C→XT
OH
H3C
The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition
and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the
second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The
product is a ß-hydroxy carbonyl compound.
base
:0:
OH
H
H
Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB
dehydration to give an a,ß-unsaturated carbonyl compound.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instruct ns
H3C
heat
OH
H3C
:0:
H
+ H₂O
H
2
Moin
H3C
H
H₂C
C⇒x=
base
:0:
OH
Hori
H3C
H.
The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and
a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second
partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a
B-hydroxy carbonyl compound.
Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB
dehydration to give an a,ß-unsaturated carbonyl compound.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
H₂C
Ἡ
:0:
heat
H
Home
H3C
+ H₂O
Why is a strong acid (like HCI) used in a Grignard reaction?
O 1)
A strong acid is used in the Grignar reaction in order to protonate the
carbonyl in order to make a better leaving group, OH. Once teh OH leaves,
the alcohol group is reformed due to water.
A stable tetrahedral intermediate with a negatively charged oxygen forms
O 2)
that does not possess a leaving group. This intermediate will exist until a
proton source from the strong acid protonates the negatively charged
oxygen, affording the neutral alcohol product.
3)
The use of a strong acid is not necessary. It is simply recommended as it
increases the yield produced in the reaction.
4)
The use of a strong acid is necessary for the formation of a Grignard
reagent. Without a strong acid, the organomagnesium compound cannot be
formed.
Chapter 22 Solutions
Organic Chemistry (9th Edition)
Ch. 22.2A - Prob. 22.2PCh. 22.4 - Without looking back, propose a mechanism for the...Ch. 22.4 - Prob. 22.8PCh. 22.4 - Prob. 22.9PCh. 22.5A - Prob. 22.10PCh. 22.5A - Prob. 22.11PCh. 22.5B - Prob. 22.12PCh. 22.5B - Predict the products of the following reactions....Ch. 22.5B - Which compounds will give positive iodoform tests?...Ch. 22.5C - Propose a mechanism for the acid-catalyzed...
Ch. 22.5C - Acid-catalyzed halogenation is synthetically...Ch. 22.6 - Show the products of the reactions of these...Ch. 22.7A - Prob. 22.18PCh. 22.7A - Prob. 22.19PCh. 22.7A - Prob. 22.20PCh. 22.7B - Prob. 22.21PCh. 22.8 - Prob. 22.22PCh. 22.8 - Prob. 22.24PCh. 22.9 - Prob. 22.25PCh. 22.9 - Prob. 22.26PCh. 22.9 - Prob. 22.27PCh. 22.9 - Prob. 22.28PCh. 22.9 - Prob. 22.29PCh. 22.10 - When cyclodecane-1,6-dione is treated with sodium...Ch. 22.11 - Prob. 22.32PCh. 22.11 - Prob. 22.33PCh. 22.12 - Prob. 22.34PCh. 22.12 - Prob. 22.35PCh. 22.12 - Prob. 22.36PCh. 22.12 - Prob. 22.37PCh. 22.12 - Show what esters would undergo Claisen...Ch. 22.13 - Prob. 22.39PCh. 22.13 - Prob. 22.40PCh. 22.14 - Prob. 22.41PCh. 22.14 - Prob. 22.42PCh. 22.14 - Show how crossed Claisen condensations could be...Ch. 22.14 - Prob. 22.44PCh. 22.14 - Prob. 22.45PCh. 22.15 - Prob. 22.46PCh. 22.16 - Prob. 22.47PCh. 22.16 - Prob. 22.48PCh. 22.17 - Prob. 22.49PCh. 22.17 - Prob. 22.50PCh. 22.17 - Prob. 22.51PCh. 22.18 - Prob. 22.52PCh. 22.18 - Prob. 22.53PCh. 22.18 - Prob. 22.54PCh. 22.18 - Prob. 22.55PCh. 22.18 - Prob. 22.56PCh. 22.19 - Prob. 22.57PCh. 22.19 - Prob. 22.58PCh. 22.19 - Prob. 22.59PCh. 22 - Prob. 22.60SPCh. 22 - 1. Rank the following compounds in order of...Ch. 22 - Prob. 22.62SPCh. 22 - Prob. 22.63SPCh. 22 - Prob. 22.64SPCh. 22 - Pentane-2,4-dione (acetylacetone) exists as a...Ch. 22 - a. Rank these compounds in order of increasing...Ch. 22 - Prob. 22.67SPCh. 22 - Prob. 22.68SPCh. 22 - 22-69 Predict the products of the following...Ch. 22 - Predict the products of these reaction sequences.Ch. 22 - Show how you would accomplish the following...Ch. 22 - Prob. 22.72SPCh. 22 - Prob. 22.73SPCh. 22 - Prob. 22.74SPCh. 22 - The Knoevenagel condensation is a special case of...Ch. 22 - Prob. 22.76SPCh. 22 - Propose mechanisms for the following reactions.Ch. 22 - Prob. 22.78SPCh. 22 - Show how you would accomplish the following...Ch. 22 - Prob. 22.80SPCh. 22 - Propose a mechanism for the following reaction....Ch. 22 - Prob. 22.83SPCh. 22 - Prob. 22.84SPCh. 22 - Prob. 22.85SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Br2, H3O+ གོ་ཤི་ན་ཤི་ H3C CH3 CH3 CH3 Aldehydes and ketones can be halogenated at their a-position by reaction with Cl₂, Br2, or 12, under acidic conditions. Using Br2 under acidic conditions, an intermediate enol is formed which adds bromine at the a-position. The reaction stops after the addition of one bromine because the electron-withdrawing halogen decreases the basicity of the carbonyl oxygen, making the protonation less favorable. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions :OH :OH Br-Br H3C H3C. Br Br CH₂ H₂ H3C CH3 H3C CH3 2barrow_forwardWhich sequence of steps will give the highest yield of a carboxylic ester product? Mix 0.1 mole of benzoic acid (C6H5CO2H) with 1.0 mol SOC12, remove unreacted SOC12, then add 0.1 mole of isopropyl alcohol O Mix 0.1 mole of benzoic acid (C6H5CO2H) with 0.1 mol of NaOH, then add 1.0 mol of isopropyl alcohol and heat, Men remove any unreacted isopropyl alcohol O Mix 0.1 mole of benzoic acid (C6H5CO2H) with 0.1 mol of NaOH, then add 0.1 mol of isopropyl alcohol and heat Mix 0.1 mole of methyl benzoate (C6H5-(C=0)-OCH3) with 0.1 mol of isopropyl alcohol and stir overnightarrow_forwardThe enolate derived from diethyl malonate reacts with a variety ofelectrophiles (not just alkyl halides) to form new carbon–carbon bonds.With this in mind, draw the products formed when Na+ −CH(CO2Et)2reacts with each electrophile, followed by treatment with H2O.arrow_forward
- Triethyloxonium tetrafluoroborate, (CH3CH2)3O+ BF4 −, is a solid with melting point 91–92 °C. Show how this reagent can transfer an ethyl group to a nucleophile (Nuc:−) in an SN2 reaction. What is the leaving group? Why might this reagent be preferred to using an ethyl halide?arrow_forwardCH3 གོན་ནི། ཡོན་ Br₂, H₂C CH₂Br H3C CH3 H₂C CH3 Aldehydes and ketones can be halogenated at their a-position by reaction with Cl₂, Br2, or 12, under acidic conditions. Using Br₂ under acidic conditions, an intermediate enol is formed which adds bromine at the a-position. The reaction stops after the addition of one bromine because the electron-withdrawing halogen decreases the basicity of the carbonyl oxygen, making the protonation less favorable. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions :0: :OH н-он H-OH₂ H₂O H₂C. H3C. CH3 CH3 H3C CH3 H3C CH3 Σαarrow_forwardGIVE THE COMPLETE PRODUCT OF THE FOLLOWING REAGENTS.arrow_forward
- The enolate derived from diethyl malonate reacts with a variety of electrophiles (not just alkyl halides) to form new carbon–carbon bonds. With this in mind, draw the products formed when Na+ −CH(CO2Et)2 reacts with each electrophile, followed by treatment with H2O.arrow_forwardGive the products of the following substitution reactions. For every reaction, show electron pairs on both nucleophile and leaving group.arrow_forwardWrite the main product by completing the reactions given below. Show the necessary mechanisms.arrow_forward
- H₂C=CHCCH3 then H3O+ The Michael reaction is a conjugate addition process wherein a nucleophilic enolate anion (the donor) reacts with an a,ß-unsaturated carbonyl compound (the acceptor). The best Michael reactions are those that take place when a particularly stable enolate anion is formed via treatment of the donor with a strong base. Alternatively, milder conditions can be used if an enamine is chosen as the donor, this variant is termed the Stork reaction. In the second step, the donor adds to the ẞ-carbon of the acceptor in a conjugate addition, generating a new enolate. The enolate abstracts a proton from solvent or from a new donor molecule to give the conjugate addition product. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions N 9aarrow_forward1. Br₂, PBrg 2. H₂O H₂C OH H3C OH Br The a-bromination of carbonyl compounds by Br₂ in acetic acid is limited to aldehydes and ketones because acids, esters, and amides don't enolize to a sufficient extent. Carboxylic acids, however, can be a-brominated by first converting the carboxylic acid to an acid bromide by treatment with PBr3. Following enolization of the acid bromide, Br₂ reacts in an α- substitution reaction. Hydrolysis of the acid bromide completes the reaction. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H3C :0: :0::Br: Br Br H3C CO-P H Br Brarrow_forwardUsing Ethylene, C2H4, and methyl bromide, CH3Br, as your sole sources of carbon, propose a synthesis for t-BuOH, (CH3)3COH. *the synthesis should take about 7 steps and will include an alkylation and a Grignard reaction. Use one equivalent of methyl bromide for the alkylation step and use the second one to make the Grignard reagent. The substrate in the Grignard reaction will be a carbonyl - make this from your alkyne AFTER alkylation. *show all reactants and intermediates H +2 H H Br -7 steps H₂C H₂C OH CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License