
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.23SP
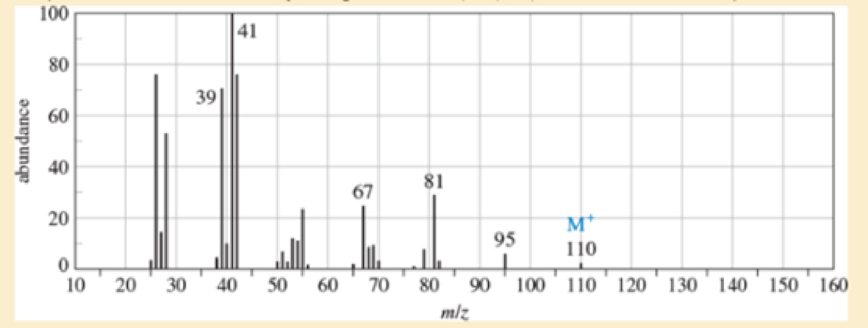
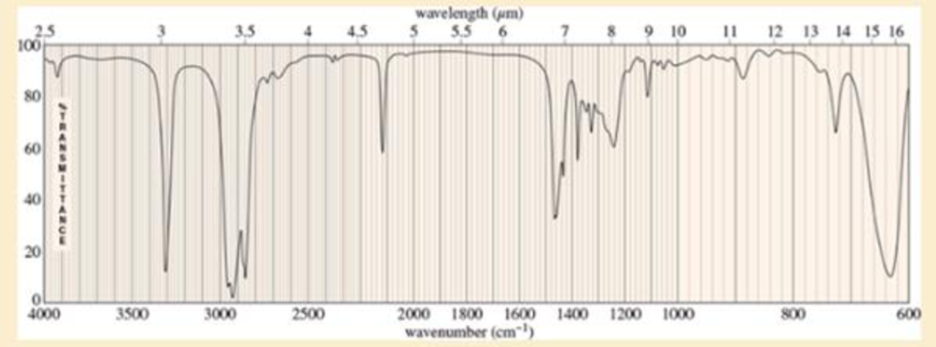
An unknown, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.
- a. Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?
- b. Use the IR spectrum to determine the
functional group (s), if any. - c. Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew that hydrogenation of the compound gives n-octane, would the structure still be uncertain?
- d. Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
True or False
1. A molecule that is "IR inactive" means that it does not produce any signal due to no vibration.
2. Infrared spectroscopic data is reported in wavenumber (cm-1) against absorbance because they have a linear relationship.
3. The signals observed from a molecule of chloropropane will have a higher wavenumber than iodopropane.
4. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than the C-C bond in an alkyne.
A) Using the mass spectrum and the IR spectrum, identify any heteroatoms in the compound. Explain your reasoning in a sentence or two.
B) Using the mass spectrum, deduce the molecular formula for the compound. Show your calculations.
C) Using the IR spectrum, identify all significant IR peaks between 4000 and 1500 cm^-1 . List these peaks and beside each one write the type of bond that gave rise to the peak.
D) Propose several structures for compound 1 consistent with your answers to part A-C.
Step 5: Draw the structure that best fits the mass spec data.
Let's review the results of the previous steps.
Step 1: The molecular ion is 27.
Step 2: There are no Cl or Br atoms based on isotope pattern.
Step 3: The molecular ion is odd, so there is one nitrogen.
Now consider Step 4 as it relates to the unknown compound.
Step 4: examine the largest peaks and calculate the difference from the molecular ion. Look for common alkyl fragments.
The mass spec data shows only one base peak at m/z 27, and a smaller peak at 26, so there are no alkyl fragments. One nitrogen
atom will have a molecular weight of 14, leaving 13 amu for the remaining unknown portion. A molecular weight of 13 amu
can only correspond to one carbon atom and one nitrogen atom, giving the molecular formula of CHN.
Deduce the structure of the unknown compound.
Select
Draw
Rings
More
Erase
H
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Look at the structure of ethyl acetate in your notebook. In which region of the 13C spectrum of ethyl acetate would you NOT expect any peaks? a.0 - 50 ppm b.50 - 100 ppm c.100 - 160 ppm d.160 - 220 ppmarrow_forwardHow can you distinguish aldehydes, esters and carboxylic acids using IR spectra? Explain using specific examples.arrow_forward1. How can you distinguish aldehydes, ketones, and carboxylic acids from each other using IR spectra? Explain, using specific examples. 2. How can you distinguish aldehydes, esters, and carboxylic acids using IR spectra? Explain, using specific examples. 3. How can you distinguish an alcohol, a primary amine, and a secondary amine using IR spectra? Explain, using specific examples. 4. How can you distinguish a terminal alkyne and a nitrile using IR spectra? Explain, using specific examples.arrow_forward
- n, foul-smelling hydrocarbon gives the mass spectrum and infrared spectrum shown.(a) Use the mass spectrum to propose a molecular formula. How many elements of unsaturation are there?(b) Use the IR spectrum to determine the functional group(s), if any.(c) Propose one or more structures for this compound. What parts of the structure are uncertain? If you knew thathydrogenation of the compound gives n-octane, would the structure still be uncertain?(d) Propose structures for the major fragments at 39, 67, 81, and 95 in the mass spectrum.arrow_forwardExplanation/Answer cannot be hand-drawn! Must be typed or illustrated digitally Analyze IR data: Identify peaks in the IR spectrum and identify their corresponding functional groups within the sample molecule. The molecules identity is given with the IR data. Analyze H NMR: Identify which hydrogens the peaks resemble. Identify their location in the molecule along with their shift, splitting, and integral. The molecules identity is given with the H NMR.arrow_forwardIndicate the functional groups found in the IR signals of: 1. Hexene 2. Cyclohexene 3. Toluene 4. Benzenearrow_forward
- Figure 1.9: The structure of this acid (C5H10O2) is: 4 singlet triplet 3 doublet 3 n=8 Figure 3: ¹H-NMR of unknown ether C (molecular formula C5H12O). Figure 1.10: The structure of this ether (C5H10O2) is: Figure 1.11: The structure of this compound (C7H16) is: мий doublet n=8 ми ми doublet triplet 1arrow_forward3. How does the infrared spectrum of methylenecyclohexane differ from 1-methylcyclohexene?arrow_forwardthe mass spectrum, IR and 13 C and 1 HNMR spectra for an unknown organic molecule. Determine the structure ofthe molecule.arrow_forward
- In IR absorption spectra of a compound, strong absorptions at 3300 cm and 2100 to 2260 cm were observed. What functional group might the compound contain? a. Alkyne b. Alkene с. Alcohol d. Carboxylic acidarrow_forwardDraw all the constitutional isomers of octane. Name each one. For each isomer: a. State the number of different sets of carbon in the structure b. State whether each set of equivalent carbons would produce an up or down signal in the DEPT-135 NMR experiment. c. State whether each set of equivalent carbons would produce a singlet, doublet, triplet, or quartet in the Off-Resonance Decoupled Experimentarrow_forward2) On spectra 2 are the mass, IR and 13 C and 1 H NMRspectra of an organic compound.a) From these spectra, determine the structure of the molecule. Rememberto ignore the triad in the 13 C NMR spectrum at 7 ppm that comes from theNMR solvent. b) Draw the structure of the molecule and label each hydrogen with a letter(A, B, C...). Then fill in the peak assignment table below. hydrogen chemical shift integration splitting pattern couples toarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY