
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.17SP
Predict the masses and the structures of the most abundant fragments observed in the mass spectra of the following compounds.
- a. 2-methylpentane
- b. 3-methylhex-2-ene
- c. 4-methylpentan-2-ol
- d. 2-methyl-1-phenylpropane
- e. cyclohexyl isopropyl ether [cyclohexyl—O—CH(CH3)2]
- f. CH3CH2CH2NHC(CH3)3 tert-butylpropylamine
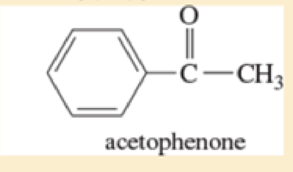
- g. *3-bromo-2-methylpentane
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Account for the formation of the base peaks in these mass spectra.
Q.) Isobutylmethylamine, m/z 44
. Cleavage alpha to C=O is prominent in ketones that usually yields a resonance-stabilized
A. Acyl ions
B. Acylium ions
C. Carbonium ion
D. Carbanion
2. fragmentation in mass spectrum is based on the following considerations, EXCEPT
A. Octet rule
B. Electronepositivity
C. Resonance
D. Hyperconjugation
How many signals would you expect to observe in the 'C NMR spectra of
b. cis-1,3-dimethylcyclohexane
CH3
a. methyl propionate
`OCH3
CH3
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
Characterize each of the following structures as aromatic, nonaromatic, or antiaromatic:
Answer: _____
Organic Chemistry As a Second Language: Second Semester Topics
Fully developed conditions are known to exist for water flowing through a 25-nim-diameer tube at 0.01 kg/s and ...
Fundamentals of Heat and Mass Transfer
Determine the number of protons, neutrons, and electrons in the following atoms: a. a hydrogen atom that has a ...
General, Organic, and Biological Chemistry (3rd Edition)
The method to determine the volume of a powered solid, liquid and a rock needs to be determined. Concept introd...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What cations are formed in the mass spectrometer by a cleavage of each of the following compounds? b. CH3CH,CH2CH,CH,CH,OH c. CH;CH,CH,CHO а. CH;CH2arrow_forward1. An aliphatic ketone absorbs at 1,715 cm-1. What is the frequency of this vibration in hertz, which is cycles per second or just per second, reciprocal seconds? 2. What is the energy equivalent of this stretching vibration in kcal/mole? 3. Why does 3,4-diethyl-3-hexene not have a carbon to carbon double bond stretching absorption band? 4. Why does a carbon to oxygen double bond absorption band have a greater intensity than a carbon to carbon double bond absorption band? 5. Using only IR, explain in detail how one could most easily differentiate between oct-1-ene and oct-1-yne if all carbon to carbon bonds are ignored. 6. Using only IR, explain in detail how one could most easily differentiate between butan-1-ol and butanoic acid.arrow_forwardWhat cations are formed in the mass spectrometer by a cleavage of each of the following compounds? os se on OH a. b. C.arrow_forward
- An oxygen-containing compound shows an absorption band at 1700 cm', and no absorption at 3300 cm', 2700 cm', or 1100 cm1. Which class of compounds is suggested in the given information? A. Carboxylic Acid B. Ester C. Ketone D. Aldehyde E. Alcoholarrow_forwardPredict the masses and the structures of the most abundant fragments observed in the mass spectra of the followingcompounds. 3-bromo-2-methylpentanearrow_forwardHalogenated compounds are particularly easy to identify by their mass spectra because both chlorine and bromine occur naturally as mixtures of two abundant isotopes. Recall that chlorine occurs as 35Cl (75.8%) and 37Cl (24.2%); and bromine occurs as 79Br (50.7%) and 81Br (49.3%). At what masses do the molecular ions occur for the following formulas? What are the relative percentages of each molecular ion? (a) Bromomethane, CH3Br (b) 1-Chlorohexane, C6H13Clarrow_forward
- 1.When zg of a certain hydrocarbon was burnt completely 0.62g of co2 and 0.25g of H2O Was obtained te emperical formular of the compound is A.CHZO B.CHZ C. CH D.C2H2 2.The atomic number of iron is 24 what is the correct electronic configuration of FE3+ 3.The shape of PCLS is 4.The function of electron gun in the mass spectrometer is A.Fires fast moving electrons B.lonize gasoeus atoms producing +ve ions C.Accelerates the ionize gasoeus atoms D.Vapourise the element 5.The coordination number and oxidation state of element E the complex K3(E(CN6) A.4 and +2 B.4 and +3 C.6 and +2 D.6 and +3 6.A substance that gives a green flame on tes coulour on heating produced an acidic gas and another gas that rekindled a glowing splint which of the following could it be A.Barium nitrate B. Barium carbonate C. Copper caronate D.Barium nitratearrow_forwardPent-1-ene and pent-1-yne are low-boiling hydrocarbons that have differentmolecular ions in their mass spectra. Match each hydrocarbon to its massspectrum.arrow_forwardWhat distinguishes the mass spectrum of 2,2-dimethylpropane from the mass spectra of pentane and isopentane?arrow_forward
- An unknown compound, B, has the molecular formula C7H12. On catalytic hydrogenation 1 mol of B absorbs 2 mol of hydrogen and yields 2- methylhexane. B has significant IR absorption band at about 3300 and 2200 cm 1. Which compound best represents B? A. 5-methyl-1,3-hexadiene B. 5-methyl-2-hexyne C. 5-methyl-1-hexyne D. 2-methyl-1,5-hexadiene OE. 3-methyl-1-hexynearrow_forwardį. A sample of edible oil was subjected to analysis using mass spectrometry (MS). The following mass spectra (X and Y) were obtained. The spectra represent 2,4- decadienal and squalene. Identify the spectrum of 2,4-decadienal and squalene and explain your answer. H3C. H 2,4-decadienal Squalene 81 0.2- 0.1 -41 67 95 1231 L 10352 100 200 300 400 500 m/z X 0.2- 69 0.15 - 81 0.1 - 0.05 .41 95 10949,7520sg83P8732 334136BDE234494891 100 200 300 400 500 m/z Y Scaled Intensity Scaled Intensityarrow_forwardWhich compound gives rise to a major [M − 18] peak in the mass spectrum? Group of answer choices A. 1-bromoheptane B. 4-methylheptane C. 2-heptanol D. 3-ethylheptanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY