
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.14SP
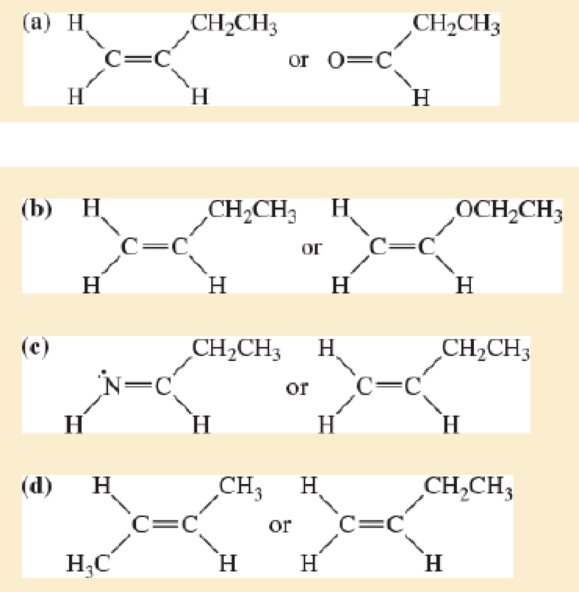
All of the following compounds absorb infrared radiation between 1600 and 1800cm−1. In each case,
- 1. show which bonds absorb in this region.
- 2. predict the approximate absorption frequencies.
- 3. predict which compound of each pair absorbs more strongly in this region.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A compound with molecular formula C4H6O gives the infrared spectrum shown here. Identify the compound.
A typical 1"B – 'H bond has a stretching frequency of 2400 cm1. Based on your understanding of
isotopic effects on vibrational frequencies, and their connection to bond strength, rank the B - H bonds
from weakest to strongest. Consider the most common isotopes of the B- H bonds highlighted below.
Make sure to explain your ranking.
10В — 1н, 10В — ?н, 11в — 1Н, 11В - н
4. As the energy gap between the HOMO and LUMO orbitals of a molecule decreases, the molecules'
electronic absorption spectrum (UV-vis spectrum) will have a larger Amax (lamba max) value. If
compound X has a Amax = 388 nm, and compound Y has a max = 442 nm, which compound has more
extensive conjugation? Place the name of the compound in the box below.
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
1. What did each of the following scientists contribute to our knowledge of the atom?
a. William Crookes
b. E...
Chemistry For Changing Times (14th Edition)
Write a Lewis formula for each of the following organic molecules: C2H3Cl (vinyl chloride: starting material fo...
Organic Chemistry - Standalone book
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- True or False 1. A molecule that is "IR inactive" means that it does not produce any signal due to no vibration. 2. Infrared spectroscopic data is reported in wavenumber (cm-1) against absorbance because they have a linear relationship. 3. The signals observed from a molecule of chloropropane will have a higher wavenumber than iodopropane. 4. The signals observed from the C-C bond in an alkene will report at a higher wavenumber than the C-C bond in an alkyne.arrow_forward3. How does the infrared spectrum of methylenecyclohexane differ from 1-methylcyclohexene?arrow_forward4.) a.) Given that the condensed molecular formula for the following compound, CSH120, predict the bonds you would expect this molecule to have based on the given IR spectrum. Draw arrows to the peaks with the bond(s) they represent. b.) Based on your findings from the IR spectrum, propose 3 different constitutional isomers that are consistent with your findings. 3000 ecoo 1000 sto 1380 34 1343 62 509 77 3346 3336 10 10 2066 12 3017 36 3326 10 2969 2912 1274 68 1236 70 1203 3007 35 49 GO 2006 1468 1117 1077 25 104 00 130 67 20arrow_forward
- Predict and compare IR stretching frequencies (cm-1) for each pair of compounds below .arrow_forward4-Explain how you could use IR spectroscopy to distinguish between compounds X, Y, and Z. CH₂ H₂C CH3 888 CH₂ X Zarrow_forwardWhich statement best describes the following pair of compounds? C4H6 and C6H12 A.) Circulated functional groups do not show absorption in the infrared spectrum because they have the same mass B.) The reduced mass of both compounds is the same so they do not absorb IR (infrared) energy. C.) The functional group specified in both compounds does not undergo changes in the dipole moment due to symmetry D.) Both represent an absorption band around 2000 cm ^ -1arrow_forward
- Draw what you expect the 1H spectra of the four compounds to be.arrow_forwardBelow are three MS spectra, Spectra 1, 2, and 3. Each of this mass spectrum corresponds to either Compound A (contains one Cl in the molecular formula), Compound B (one I in the molecular formula), and Compound C (one Br in the molecular formula). Identify which spectra corresponds to which compound. Provide explanations for your choices.arrow_forward8. Which of the following pairs of compounds is likely to absorb radiation at the longer wavelength and with greater intensity? (a) CH3CH2CO₂H or CH2=CHCO₂H (b) CH3CH=CHCH=CHCH3 or CH3C = C—C = CCH3 OCH3 (c) or CH3arrow_forward
- 12) infrared spectra and 1H NMR spectra for each compound are provided on this and the next Compounds A, B, and C were found to have a molecular formula CaHsO2. The page. Determine the structural formula of each compound. IHD = Compound A 6 H Compound A 1 H 11 10 Compound B 3 H Compound B Kexchanges with D20) 2 H 1 H 10 Compound C ff 口 Compound C 3H 1 H 2H 2 H 10arrow_forward1. The energy of a photon is. proportional to its wavelength and proportional to its frequency. (directly or indirectly) 2. Which of the following has a lower characteristic stretching frequency, the C O bond or the C-O bond? Explain briefly. C=0 3. Ethyne HCECH does not show IR absorption in the region 2000-2500 em-! because. 4. Which statement best explains why a carbonyl absorbs infrared radiation at a higher frequency than an alkene absorption?arrow_forwardational nown b elow. Vibrations of XEC14: Left: Scissoring. Middle: Wagging. Right: Twisting. Which of these motions will be IR active? In other words, which of these motions will lead to the absorption of IR light and be represented by a peak in the IR spectrum of this compound? O A. Only twisting is IR active. O B. Only wagging is IR active O C. Both scissoring and twisting are IR active. D. Both wagging and twisting are IR active. O E. All of these vibrations are IR activearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY