
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 15.28SP
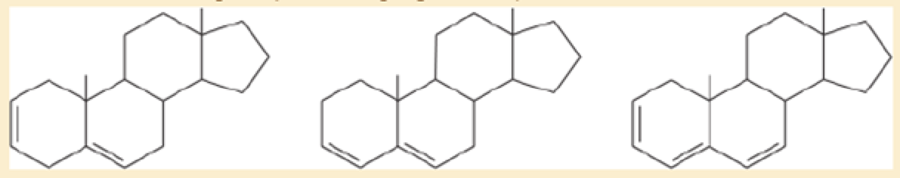
A solution was prepared using 0.0010 g of an unknown steroid (of molecular weight around 255) in 100 mL of ethanol. Some of this solution was placed in a 1-cm cell, and the UV spectrum was measured. This solution was found to have λmax=235nm, with A=0.74.
- a. Compute the value of the molar absorptivity at 235 nm.
- b. Which of the following compounds might give this spectrum?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
12. The mass spectrum of 2,2-dimethylpropane shows only a very weak molecular ion peak at m/z = 72. However, a large peak at m/z = 57 is seen. Suggest a possible structure of the fragment giving rise to this large peak and suggest a reason as to why this peak is so large.
type answer
A solution was prepared using 0.0010 g of an unknown steroid (of molecular weight around 255) in 100 mL of ethanol.Some of this solution was placed in a 1-cm cell, and the UV spectrum was measured. This solution was found to havelmax = 235 nm, with A = 0.74.(a) Compute the value of the molar absorptivity at 235 nm.(b) Which of the following compounds might give this spectrum?
The molecular ion region of the spectrum of biphenyl. M1 is observed at m/z 154 and the intensity of M 1 1 is 12.9% of M1. What formulas of the type CnHxOyNz are consistent with the spectrum?
Chapter 15 Solutions
Organic Chemistry (9th Edition)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12. The mass spectrum of 2-methylhexane is shown below. What is the m/z value of the M* peak and of the base peak? Give possible structures of the fragments giving rise to the large peaks at m/z = 85,57, and 43. 100 - 80 - 40 20 50 60 70 80 90 100 10 20 30 40 m/z Relative Intensityarrow_forward750 400 11. Short answer. Refer to the color wheel on the right. If a solution is illuminated with white light and absorbs all wavelengths between 480 and 560 nm, what color would it appear to be? Violet Red 430 630 Blue Orange 480 590 Green Yellow 560 12. Short answer. You will need the color wheel on the right. Look at the absorption spectrum of the solution shown on the right. What color would this solution be? 800 13. Short answer. Look at the image of a spectrophotometer on the right. Structures/knobs A-E are labeled. Which knob will you have to use to zero the instrument itself? 20D BIOLOG. 14. Short answer. According to Beer's Law, given that Molar Absorptivity and path length is usually constant, Absorbance A is directly proportional to 15. Choose the one answer that fits best. Which of these is not standard components of a spectrophotometer? a. A sample chamber to hold your tube with sample solution b. A light source c. A prism or grating to filter out certain wavelengths of…arrow_forwardQ1. Why is it important to acquire a reference spectrum and subtract it from the sample’s spectrum? Q2. Why are quartz cuvettes better for UV-visible absorption spectroscopy than polystyrene cuvettes, especially at lower wavelengths (UV)? Q3. What would happen if you put too much of a compound in the cuvette during the spectral measurement? Q4. Why is it important to avoid any scratch on the cuvette? Q5. Could you use a compact fluorescent light (CFL) bulb to perform UV-visible absorption spectroscopy? Why/why not? Q6. Predict the maximum absorption wavelength for 2,4-heptadien-6-one using Woodward-Fieser rules. Q7. Why are n-π* transitions generally of low intensities/low absorptions? Q8. What photon energy and wavelength would you need to send on a compound having an energy gap (Eg) of 2.7 eV? Detail your answer with appropriate information. Q9. Using molecular orbital theory/concept (draw the important molecular orbitals), explain the difference in absorption for the…arrow_forward
- The M+ peak is at 136. What does is mean to have the [M+2] peak at 138? What is the chemical formula and the structure for the M+ peak? What are the structures for the peaks at 57, 43, and 29 m/z?arrow_forward1. True or False a. UV-Vis spectroscopy normally reports data in the from of bands rather than single peaks because of overlapping electronic transitions that are being recorded by the detector. (T/F) b. When using linear regression to translate absorption data using Beer's Law, the y-intercept (+b) of the linear equation represents the path length. (T/F) c. Phosphorescent materials give a glowing effect because the electrons remain at an excited state for much longer, which is the cause for the "glow". (T/F)arrow_forwardBelow is the Electrospray ionization mass spectrum of a compound; what is the exact mass of this compound? 100- 80 60 40 20 10 95 96 97 98 MS-NW-5593 20 fpoy.ql.ppppplıyo 30 40 50 60 70 80 90 100 110 TTTTT 120arrow_forward
- 4. Identify ketone for this spectrum. 100 b. 57 29 I 86 Relative abundance 80 60 40 20 20 40 60 m/z 80 100 120arrow_forwardChemistry please help me wt the ff: a. Calculate the concentration (g/mL) of acrolein [CH2=CHCHO] that should be prepared in orderto give an absorbance of 0.8 at 217 nm ( = 16,000). b. Propose a structure for C7H4O2Cl2, as an acid with λmax 242 nm. c. Describe briefly the steps in obtaining the spectrum of a sample using Thermo Scientific Nicolet IS5 FT-IR Instrument starting from loading the solid and liquid samples.arrow_forwardThe sample you analyzed has an intensity of 0.83 and a wavelength 672 nm. When you reanalyze the sample 5 minutes later, the sample has an intensity of 0.73 and its wavelength is 672 nm. The sample's wavelength has undergone O a bathochromic shift. a hypochromic effect. Oa hyperchromic effect. Oa hypsochromic shift.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY