
Concept explainers
Benzocaine is the active ingredient in topical pain relievers such as Orajel and Anbesol. Give the molecular formula for benzocaine.
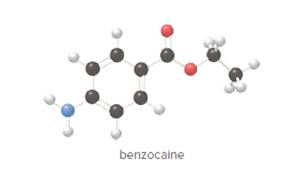
- Give the molecular formula for benzocaine.
- Draw in all lone pairs on heteroatoms using a skeletal structure.
- How many trigonal planar carbons does benzocaine contain?
- Predict the water solubility of benzocaine.
- Label all polar bonds.
(a)
Interpretation:
To determine the molecular formula of benzocaine from the ball and stick model.
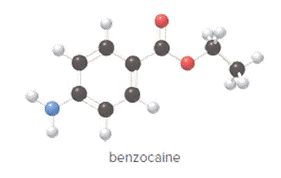
Concept Introduction:
In ball and stick model, black color ball represents carbon atom, white color ball represents hydrogen atom, red color ball represents oxygen atom and blue color ball represents nitrogen atom. To determine the molecular formula, convert the ball and stick model to complete structure.
Answer to Problem 81P
Molecular formula of benzocaine is
Explanation of Solution
The compound is as follows:
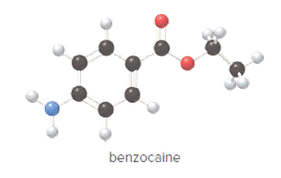
Convert ball and stick model to normal structure. Replace black balls by carbon atoms, red balls by oxygen atoms, blue ball by nitrogen atom and whit balls by hydrogen atom. But, all the bonds between atoms are same.
In benzocaine, nine carbon atoms, eleven hydrogen atoms, two oxygen atoms and one nitrogen atom present. Hence, molecular formula of benzocaine is
(b)
Interpretation:
To draw all lone pairs on heteroatoms of benzocaine using a skeletal structure.
Concept Introduction:
Heteroatoms are those atoms in an organic compound other than carbon and hydrogen atom like oxygen, nitrogen, etc. Lone pairs are pair of electrons available on an atom after bond formation.
Answer to Problem 81P
The structure of benzocaine having all lone pairs on heteroatoms is as follows:
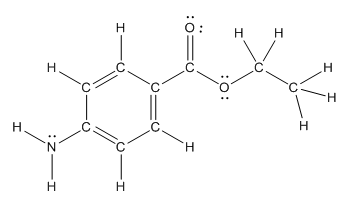
Explanation of Solution
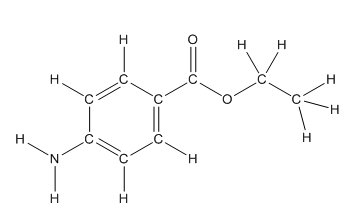
Skeletal structure of benzocaine is as follows:
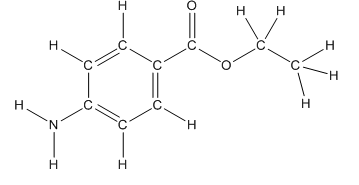
The heteroatoms present in benzocaine are nitrogen atom and two oxygen atoms. Nitrogen has five valence electrons. Out of five electrons, two electrons are used for making two (N-H) bonds, one electron is used for making one N-C bond. Remaining two electrons forms a lone pair. Oxygen has six valence electrons. For double bonded oxygen atom, two electrons are used for making the double bond. Remaining four electrons forms two lone pair. Other oxygen atom forms two bonds with carbon atoms means two electrons are used in bond formation. So, two pairs of lone pair present. Hence, the structure having all lone pairs on heteroatoms is as follows:
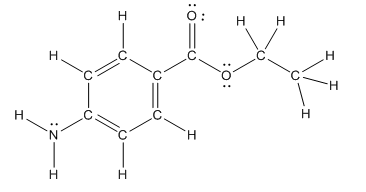
(c)
Interpretation:
To determine the number of carbon atoms having trigonal planar shape in benzocaine.
Concept Introduction:
The following table should be used while determining shape around an atom.
| Number of groups | Number of atoms | Number of lone pairs | Shape | Bond angle |
| 2 | 2 | 0 | Linear | |
| 3 | 3 | 0 | Trigonal planar | |
| 4 | 4 | 0 | Tetrahedral | |
| 4 | 3 | 1 | Trigonal pyramidal | |
| 4 | 2 | 2 | Bent |
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Answer to Problem 81P
In benzocaine, seven carbon atoms have trigonal planar structure.
Explanation of Solution
If an atom is surrounded by three groups, then the shape around that particular atom is trigonal planar.
Structure of benzocaine is as follows:
To find the number of carbon atoms having trigonal planar shape in benzocaine, observe the carbon atoms and find which carbon atom has three groups surround it.
All the carbon atoms in the benzene ring have three atoms around them also the carbon bonded to the benzene ring has three groups surround it. So, seven carbon atoms in benzocaine have trigonal planar structure.
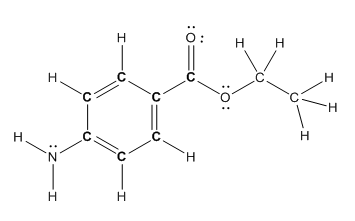
The bold carbon atoms have trigonal planar structure.
(d)
Interpretation:
To predict the solubility of benzocaine in water.
Concept Introduction:
Water is a polar solvent. To dissolve in water the compound must be polar. Polar compound is that compound in which polar bonds are present. The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 81P
Benzocaine is water soluble compound.
Explanation of Solution
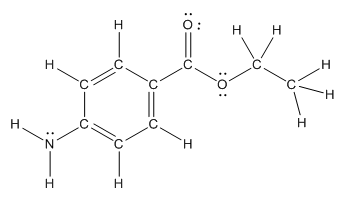
The structure of benzocaine is as follows:
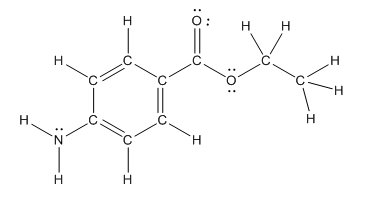
As the compound contains heteroatoms like nitrogen, oxygen the compound is polar that is, they can form hydrogen bonds with water. So, benzocaine is water soluble compound.
(e)
Interpretation:
To label all the polar bonds in benzocaine.
Concept Introduction:
The unequal sharing of valence electrons in a bond is called polar bond. Polar bond result when the bond formed between two atoms in which one atom is more electronegative than the other one. One example of polar bond is
Structure of HCl is as follows:

In
Answer to Problem 81P
Polar bond in benzocaine are as follows:
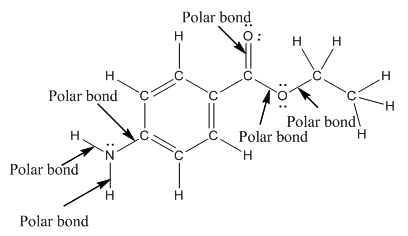
Explanation of Solution
The structure of benzocaine is as follows:
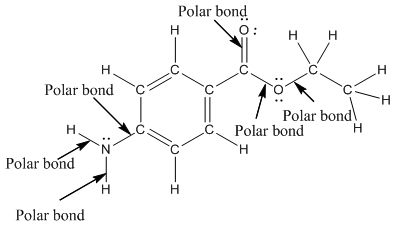
In organic compound, most of the polar bonds formed between carbon and heteroatoms like oxygen, nitrogen, sulphur etc. In benzocaine six polar bonds present that is, three polar bond between carbon and oxygen (oxygen is more electronegative than carbon), two polar bond between nitrogen and hydrogen (nitrogen is more electronegative than hydrogen) and on polar bond between carbon and nitrogen (nitrogen is more electronegative than carbon).
Want to see more full solutions like this?
Chapter 11 Solutions
General, Organic, and Biological Chemistry - 4th edition
- 1. Use the image provided to answer the question in detail: a) Two of the earliest drug molecules featured in Chapter 8 are Vicodin and Heroin, whose chemical structures are very similar. Both of these molecules contain an amine functional group and several rings. Indicate the number of aromatic rings in each structure, and identify the amine functional group in each molecule as primary, secondary, or tertiary.arrow_forwardIdentify and label all functional groups (alcohols, amines, amides, carboxylic acids, ketones, aldehydes, aromatic rings, aromatic amines, etc) of Carvone on the Lewis Structure. You do not have to circle alkanes. Make sure to add additional details about the functional groups of Carvonearrow_forwardIn a laboratory experiment, one sherbet lemon sweet produced 6.00 mL of carbon dioxide. d) Calculate the minimum mass (g) of tartaric acid necessary to produce this 6.00 mL volume of carbon dioxide. (Assume that 1 mole of carbon dioxide occupies 24.0 L at room temperature and pressure). e) By making the appropriate substitutions for tartaric acid's H (hydrogen) and OH (hydroxyl) groups among positions A, B, D and E in the structure shown below, how many different stereoisomers of tartaric acid are possible? HO₂C B CO₂H A Earrow_forward
- sont HO OH Alcohol Carboxylic acid Amine C. Lone pairs and formal charges. OH H Amide Aldehyde Amine, Alcohol Carboxylic acidarrow_forward1. Draw the Full Lewis chemical structure of a simple ester of your choice containing exactly five carbon atoms.arrow_forwardDirections: Write the correct Lewis structure and show the step-by-step solution on how you came up with the structure. HCN - highly toxic conjugate acid of a cyanide that is used as a chemical weapon agentarrow_forward
- Determine whether the statement is true or false. Defend your answer with a MAXIMUM of 3 sentences. 1.Carboxyl groups are one of the most polar functional groups. Acetic acid (vinegar) has carboxyl group, so it has the most solubility with water.arrow_forwardUnlike methanol, which is a nearly odorless liquid, methanethiol (CH3SH) is a gas with an appalling odor reminiscent of skunks. It is one of the compounds added to natural gas as a warning for gas leaks. Using appropriate drawing tools, draw the structure of methanethiol in such a way that there is double bond between carbon and sulfur.arrow_forwarddraw complete Lewis structures for both the reactants ethanol and acetic acid and the products ethyl acetate and water. Include lone pairs when needed.arrow_forward
- What is Resonance Theory? Sate five conclusions that can be drawn from the theory. State the two main experiments that were used to establish the extra stability of the benzene molecule. What are the factors that confer Aromaticity to an organic molecule?arrow_forwardH3C H3 Metaphosphoric Acid CH3 KOCH3, CH3OHarrow_forward1. Predict the structure of ethanol once it oxidizes in the human body. Draw the condensed structure. 2. A student found a bottle in the lab filled with a colorless solution. The label on the bottle was fading. The students were able to correctly identify, but knewit was aa phenol from the readable part of the label. The student decided to run a ferric chloride test. The test resulted in a greed color. What is the identity of the liquid? 3. Predict below alcohols their skeletal structure; if soluble in water; does it contain a phenol group, is it primary, secondary, or tertiary alcohol; can it be oxidized by chromate; if yes what is the oxidation product and draw the skeletal structure; will the alcohol form a colorful ccomplex with Fe3+? Thymol ( found in oil of thyme, antiseptic properties) Menthol ( found in mint, gives a cooling sensation) Cinnamyl aalcohol (used in perfumes ans deodorants) Cety alcohol (ingredient in shampoo, creams, and lotions)arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





