
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 13E
Interpretation Introduction
Interpretation: The reason behind the representation of allene on the right more accurate than the one on the left in below figure should be explained.
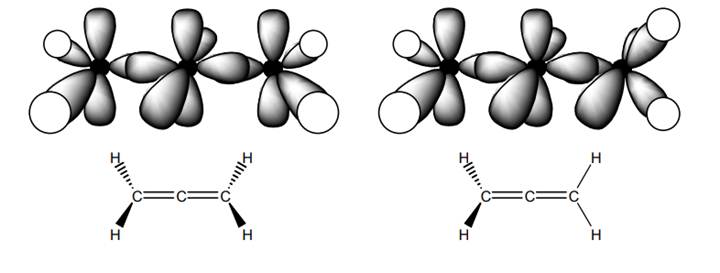
Concept introduction: Bonds formed due to head-to-head overlap are termed sigma bond while ones formed by sideways or lateral overlap are named pi-bonds.
The planar system or‘
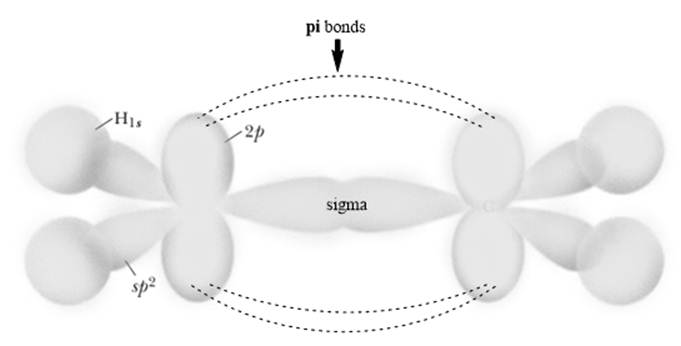
Each carbon in ethylene is trigonal planar due to
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1. CHǝMgBr
A
2. H3O+
1. KCN
B
2. H3O+
1. CH3Li
C
2. H3O+
1. (CH3)2CULI
2. H3O+
Done
Click on those molecules below which have a dipole moment.
If there are none, submit your answer without clicking.
HC
CH3
||
CH₂
Н.
H3C
CH3
'H
ه
CH₂
CI
Click on those molecules below which have a dipole moment.
If there are none, submit your answer without clicking.
CI
CH₂
CH3
CH₂
Chapter 3 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 3 In the answer boxes, indicate whether carbon 2 compound. CI G CI ||Z and carbon 3 have R or 8 configuration in the followingarrow_forwardso pls draw the flat representation, not the Chair form of the overall Most stable and least stable formsarrow_forwardI. H C/C Br F Is this molecule polar, and if so, then what is the direction of the polarity vector? Yes; towards the direction of H No; this molecule is non-polar. Yes; towards the direction of F and Cl Yes; towards the direction of F and Br Yes; towards the direction of F Yes; towards the direction of Br Yes; towards the direction of Br and Cl Yes; towards the direction of Clarrow_forward
- Rank each of the representations of NOCl from best to worst, placing the best structure on top and the worst at the bottom.arrow_forwardCANNOT BE HAND DRAWN! Please help correct my work Determine if your molecule contains any chiral carbons. If there are chiral carbons in your molecule, circle or highlight all of them. If your molecule does not contain any chiral carbons explain why none of the carbons are chiral.arrow_forwardChoose the factor that explains this observation. || A) resonance stabilization B) charge C) electronegativity D) polarizability OH OH₂arrow_forward
- Observe the Newman projection shown below. The front atoms were rotated 120° counterclockwise. Which of the following represents the correct molecule as a result of this rotation? NO2 H. Br CI CH2CH3 H CI H. Br H3CH,C NO2 H NO2 CI Br CH,CH3 CH,CH, H. Br O,N 'CIarrow_forwardDraw a molecule that contains at least one sp-hybridized carbon and at least 1 sp2- hybridized carbon. Label those two carbons (one sp- and one sp2-hybridized.)arrow_forwardTag all the sp² hybridized carbon atoms in this molecule. If there are none, please check the box below. H-C=C - H There are none.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning