
Concept explainers
Interpretation:
The pro-R or pro-S hydrogen removed from citrate during the dehydration in step 2 of the citric acid cycle.
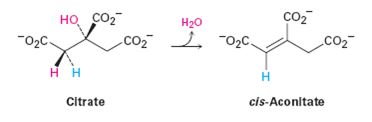
Concept introduction:
A molecule is said to be pro chiral, if it can be converted from achiral to chiral a single chemical step.
For instance, an unsymmetrical
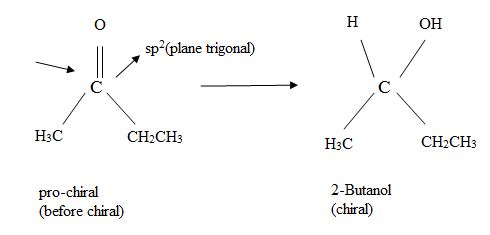
Which enantiomer of 2-butanol is produced depends on which face of the planar carbonyl undergoes reaction to distinguish between the possibilities, the stereo chemical description Re and Si (announced ‘ray’ and ‘sigh’) are used.
The concept of this designation is as follows: Rank the three groups attached to the trigonal, sp2 hybridized carbon according to the conventional Cahn-In gold-Prelog (CIP) system and imagine curved among from the highest to second highest, to third highest ranked substituents.
Trending nowThis is a popular solution!

Chapter 29 Solutions
Organic Chemistry
- The dehydration of citrate to yield cis-aconitate, a step in the citric acid cycle, involves the pro-R “arm’’ of citrate rather than the pro-S arm. Which of the following two products is formed?arrow_forwardLinoleic acid is shown below. What makes this fatty acid particularly susceptible to autoxidation? 1. The red CH bond has a low bond dissociation energy because it is doubly allylic. 2. The red CH bond has a high bond dissociation energy because it is doubly allylic. 3. The red CH bond is the most accessible to reaction with O2 because it is the least sterically crowded CH bond. 4. Both 2 and 3.arrow_forwardwhat is the heat combustion of C2H5OH at 25C?arrow_forward
- Click on the glycosidic linkage НО CH2OH ОН ОН CH₂OH ОН ОН ОНarrow_forwardDraw the alkene that is needed to synthesize glutamine by an enantioselective hydrogenation reaction using H₂ and Rh.arrow_forward5 Identify each of the following molecules as a steroid, a fatty acid, or a wax. (a) Isolated from the seeds of the jojoba plant (b) (c) HO. Erucic acid, isolated from mustard seed H Medrogestone, a synthetic drugarrow_forward
- Draw the ketohexose provided in the B-pyranose form using the pyranose skeleton as a guide. O: H +OH H +OH HO -H HO- HO.arrow_forwardin the following molecule the glycosidic bond is circled. CH2OH CH2OH H CH2OH OH HO H H НО H. ОН ÓH True Falsearrow_forward27. Which of the following statements about cholesterol is not correct? CH3 CH3 H. d но Cholesterol (a) Cholesterol is a steroid that contains a tetracyclic ring system. (b) Cholesterol is a steroid that contains 8 chiral carbons and can form 28 or 256 stereoisomers. (c) Each atom or group attached to a ring-junction carbon (i.e., carbons a -e) is in a trans or axial position. Because of this the tetracyclic ring system is mostly flat. (d) Cholesterol is used to synthesized vitamin D, bile acids, sex hormones, and adrenocorticoid hormones. (e) Cholesterol is not found in the cell membranes of animals.arrow_forward
- Digitalis is a preparation made from the dried seeds and leaves of the purple foxglove, Digitalis purpurea, a plant native to southern and central Europe and cultivated in the United States. The preparation is a mixture of several active components, including digitalin. Digi- talis is used in medicine to increase the force of myocardial contraction and as a conduction depressant to decrease heart rate (the heart pumps more forcefully but less often). HC OH H,C H CH3 H. H. (a) Describe this glycosidic bond OCH, H A (b) Draw an open-chain Fischer projection of this monosaccharide CH3 H. (e) Describe this glycosidic bond OCH, HA H. HO H) H. OH НО (d) Name this monosaccharide unit H. OH Digitalinarrow_forwardWhat epoxide is needed to convert CH3CH2MgBr to attached alcohols, after quenching with water ?arrow_forwardWhy has triglyceride autooxidation occurred selectively to give the following product? QOH O This peroxide is the most stable option. O That position is the least crowded. O Allylic H atoms are easier to abstract than alkyl. O Oxygen is a selective diradical.arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


