
Concept explainers
(a)
The ionization energies of the L, M and N shells.
(a)
Answer to Problem 38P
The ionization energies for the L shell is
Explanation of Solution
The figure 1 shows the transitions from higher levels N shell down to K shell.
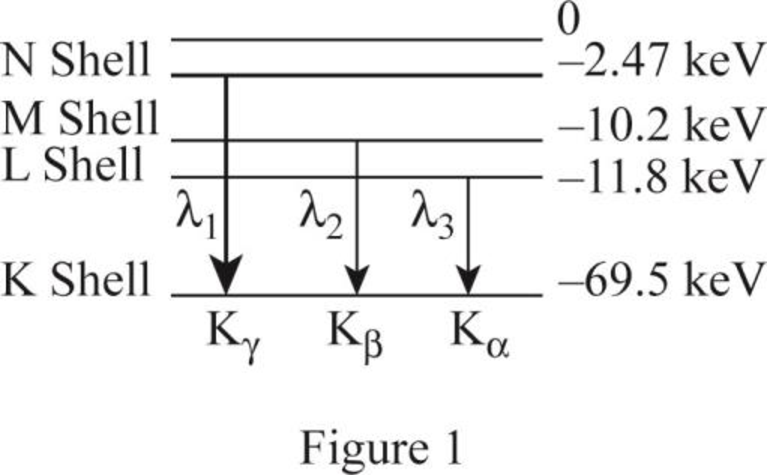
The K series includes the transition from higher levels down to the K shell
The ionization energy for the K shell is
Write the expression for energy of the photon.
Here,
Conclusion:
Substitute
Convert the energy of the photon into kilo electron volt.
Substitute
Convert the energy of the photon into kilo electron volt.
Substitute
Convert the energy of the photon into kilo electron volt.
Similarly the table 1 shows the ionization energy for the shells.
| Photon energy | transition | Energy of level | level | |
| 67.03 | N | |||
| 59.3 | M | |||
| 57.7 | L |
The ionization energy for the K shell is
Therefore, the ionization energies for the L shell is
(b)
Draw the diagram of the transition.
(b)
Answer to Problem 38P
The table 1 shows the transition of the X-ray spectrum of tungsten element.
Explanation of Solution
From (a) The table shows the transition of the X ray spectrum..
| Photon energy | transition | Energy of level | level | |
| 67.03 | N | |||
| 59.3 | M | |||
| 57.7 | L |
Conclusion:
Therefore, the table 1 shows the transition of the X-ray spectrum of tungsten element..
Want to see more full solutions like this?
Chapter 29 Solutions
Principles of Physics: A Calculus-Based Text
- The ion Li2+ makes ail atomic transition from ail n = 4 state to ail n = 2 state, (a) What is the energy of the photon emitted during the transition? (b) What is the wavelength of the photon?arrow_forwardIn extreme-temperature environments, such as those existing in a solar corona, atoms may be ionized by undergoing collisions with other atoms. One example of such ionization in the solar corona is the presence of C5+ ions, detected in the Fraunhofer spectrum. (a) By what factor do the energies of the C5+ ion scale compare to the energy spectrum of a hydrogen atom? (b) What is the wavelength of the first line in the Paschen series of C5+ ? (c) In what part of the spectrum are these lines located?arrow_forwardThe L series of the characteristic x-ray spectrum of tungsten contains wavelengths of 0.1099 nm and 0.1282 nm. The L-shell ionization energy is 11.544 keV. Which x-ray wavelength corresponds to an N → L transition? Determine the ionization energies of the M and N shells: If the incident electrons were accelerated through a 40.00 keV potential difference before striking the target, find the shortest wavelength of the emitted radiation:arrow_forward
- The Ka X-ray emission line of tungsten occurs at λ = 0.021 nm. The energy difference between K and L levels in this atoms is about (a) 0.51 MeV (b) 1.2 MeV (c) 59 keV (d) 13.6 eVarrow_forwarda. The electron of a hydrogen atom is excited into a higher energy level from a lower energy level. A short time later the electron relaxes down to the no = 1 energy level, releasing a photon with a wavelength of 93.83 nm. Compute the quantum number of the energy level the electron relaxes from, nhi. Note: the Rydberg constant in units of wavenumbers is 109,625 cm-1 nhi =16 b. What would the wavenumber, wavelength and energy of the photon be if instead no = 1 and nhi = 4? V: 6.9121e14 x (cm-¹) λ: (nm) E: 45.8e-20 ✓ (1)arrow_forwardConsider the atomic spectra for the H-atom: the Lyman series emits UV photons, the Balmer series emits visible photons, the Paschen series emits IR photons, and the Brackett series emits far IR photons. What type of photons would you expect from the next series? Briefly explain.arrow_forward
- A hypothetical atom has only two atomic energy levels, separated by 3.2 eV. Suppose that at a certain altitude in the atmosphere of a star there are 6.1 * 1013/cm3 of these atoms in the higher-energy state and 2.5 * 1015/cm3 in the lower-energy state. What is the temperature of the star’s atmosphere at that altitude?arrow_forwardA triply ionised beryllium atom (Be+++, Z = 4) has only one electron in orbit about the nucleus. If the electron decays from the n 7 level to the first excited state (n = 2), calculate the wavelength of the photon emitted. Please give your answer in units of nm, rounded to one decimal place. Answer:arrow_forwardYou measure a wavelength of 397.1 nm in a spectroscopy experiment. You identify this as a particular transition from the hydrogen Balmer series. Which transition is it?arrow_forward
- The energies for an electron in the K, L, and M shells of the tungsten atom are -69,500 eV, -12,000 eV, and -2200 eV, respectively. Calculate the wavelengths of the Ka and Kb x rays of tungsten.arrow_forwardThe light observed that is emitted by a hydrogen atom is explained by a simple model of its structure with one proton in its nucleus and an electron bound to it, but only with internal energies of the atom satisfying EH=−RH/n2EH=−RH/n2 where RHRH is the Rydberg constant and nn is an integer such as 1, 2, 3 ... and so on. When a hydrogen atom in an excited state emits light, the photon carries away energy and the atom goes into a lower energy state. Be careful about units. The Rydberg constant in eV is 13.605693009 eV That would be multiplied by the charge on the electron 1.602× 10-19 C to give 2.18× 10-18 J A photon with this energy would have a frequency f such that E=hf. Its wavelength would be λ = c/f = hc/E. Sometimes it is handy to measure the Rydberg constant in units of 1/length for this reason. You may see it given as 109737 cm-1 if you search the web, so be aware that's not joules. The following questions are intended to help you understand the connection between…arrow_forwardA visible (violet) emission spectral line for chromium (Cr) occurs at wavelength λ = 425.435 nm. A) What is the frequency (ν) of this light?(Give correct units and answer to six significant figures.) B) What is the magnitude of the energy change associated with the emission of one mole of photons of light with this wavelength?arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning




