
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 24.40SP
Lipoic acid is often found near the active sites of enzymes, usually bound to the peptide by a long flexible amide linkage with a lysine residue.
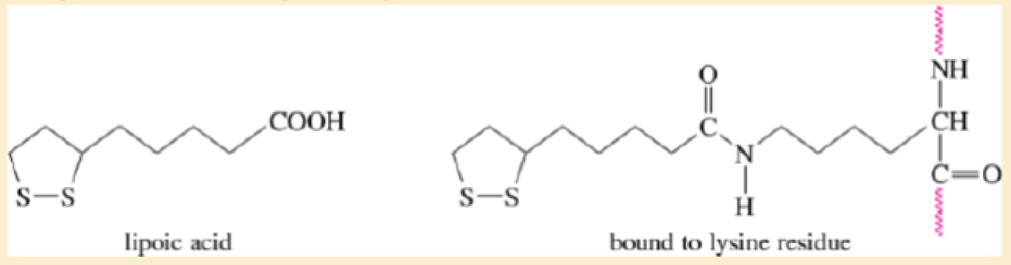
- a. Is lipoic acid a mild oxidizing agent or a mild reducing agent? Draw it in both its oxidized and reduced forms.
- b. Show how lipoic acid might react with two Cys residues to form a disulfide bridge.
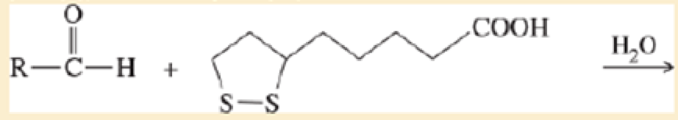
- c. Give a balanced equation for the hypothetical oxidation or reduction, as you predicted in part (a), of an
aldehyde by lipoic acid.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a. which amino acid can form an N-linked bond with any carbohydrate
b. which amino acid would most likely be found at a rigid turn in a protein structure?
D. Consider the amino acids glycine, proline and lysine.
a. How many tripeptides can be formed from these three amino acids if each is used
only once in the structure?
b. Using three-letter abbreviations for the amino acids, give the sequence of each of
the possible tripeptides.
c. Draw the structure of the dipeptide that has proline at its N-terminal amino acid
and glycine as its C-terminal amino acid. Circle each peptide bond.
Draw Tryptophan in a peptide bond. Explain all of the bonds using valence bond theory (VBT). Next, explain where VBT fails and how molecular orbital theory is a better description of key regions of this amino acid and the peptide bonds it forms with other residues
Chapter 24 Solutions
Organic Chemistry (9th Edition)
Ch. 24.2A - Draw three-dimensional representations of the...Ch. 24.2A - Prob. 24.2PCh. 24.2B - The herbicide glyphosate (Roundup) kills plants by...Ch. 24.4 - Draw the structure of the predominant form of a....Ch. 24.4 - Draw the resonance forms of a protonated guanidino...Ch. 24.4 - Although tryptophan contains a heterocyclic amine,...Ch. 24.4 - Prob. 24.7PCh. 24.4 - Prob. 24.8PCh. 24.5A - Show how the following amino acids might be formed...Ch. 24.5B - Prob. 24.10P
Ch. 24.5C - Prob. 24.11PCh. 24.5C - Show how you would use a Strecker synthesis to...Ch. 24.6 - Suggest how you would separate the free i-ammo...Ch. 24.7A - Propose a mechanism for the acid-catalyzed...Ch. 24.7A - Give equations for the formation and...Ch. 24.7B - Prob. 24.16PCh. 24.7C - Prob. 24.17PCh. 24.8B - Draw the complete structures of the following...Ch. 24.9C - Prob. 24.19PCh. 24.9C - Prob. 24.20PCh. 24.9C - Prob. 24.21PCh. 24.9E - Prob. 24.22PCh. 24.9E - Prob. 24.23PCh. 24.10A - Propose a mechanism for the coupling of acetic...Ch. 24.10B - Show how you would synthesize Leu-Gly-Ala-Val-Phe...Ch. 24.10B - Show how solid-phase peptide synthesis would be...Ch. 24 - a. The isoelectric point (pl) of phenylalanine is...Ch. 24 - Prob. 24.28SPCh. 24 - Prob. 24.29SPCh. 24 - Prob. 24.30SPCh. 24 - Prob. 24.31SPCh. 24 - Suggest a method for the synthesis of the...Ch. 24 - Prob. 24.33SPCh. 24 - Write the complete structures for the following...Ch. 24 - The following structure is drawn in an...Ch. 24 - Prob. 24.36SPCh. 24 - Prob. 24.37SPCh. 24 - Show the steps and intermediates in the synthesis...Ch. 24 - Prob. 24.39SPCh. 24 - Lipoic acid is often found near the active sites...Ch. 24 - Prob. 24.41SPCh. 24 - Prob. 24.42SPCh. 24 - Prob. 24.43SPCh. 24 - Complete hydrolysis of an unknown basic...Ch. 24 - Prob. 24.45SPCh. 24 - Prob. 24.46SPCh. 24 - Prob. 24.47SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a segment of the backbone of a protein that is long enough for three peptide linkages to be present.arrow_forwardWhy is the phrase unstructured segment of a protein somewhat of a misnomer?arrow_forward(a) How many tripeptides can be made from glycine, alanine, and leucine, using each amino acid only once per tripeptide? (b) Write the structural formulas of these tripeptides and name them in the shorthand abbreviation used for showing amino acid sequences.arrow_forward
- A. Draw the structure of L-valine in astrongly basic solution. B. What is the charge of this amino acid in a strongly basic solution?arrow_forwardDisulfide linkages: A. are covalent bonds. B. are a type of electrostatic interaction. C. occur between methionine residues. D. can be disrupted by SDS. E. can only occur within the same polypeptide chain.arrow_forwardA. Lysine is considered a basic amino acid containing a guanidino group. If all ionizable protons from lysine were deprotonated, what will be the overall charge for the lysine? B. Which of the following is true regarding amino acids? -Leucine and isoleucine have the same molecular mass. -Glycine is a chiral molecule. -Proline is considered to be an ⍺-amino acid. -Lysine (short for L) is a basic amino acid.arrow_forward
- ION Glycine molecule shows a large conductivity when dissolved in water because; A. В. Glycine dissociates directly and form Glycine ionizes internally forming a amino and carboxylate ions dipole of two charge centers D. Glycine ionizes to form a positive ion by Glycine ionizes to form a negative ion by protonation of amino group deprotonation of carboxylic group Select answer Save C.arrow_forwardGive the amino acid sequence of each peptide using the fragments obtained by partial hydrolysis of the peptide with acid. a. a tetrapeptide that contains Ala, Gly, His, and Tyr, which is hydrolyzed to the dipeptides His-Tyr, Gly-Ala, and Ala-His b. a pentapeptide that contains Glu, Gly, His, Lys, and Phe, which is hydrolyzed to His-Gly-Glu, Gly-Glu-Phe, and Lys-Hisarrow_forwarda-Amino acids have the following general form: H H,N-C R-group a) Draw the structures of any amino acid (one for each of the following) with: i. A hydrophobic R-group-that contains a double bond An acidic R-group A basic R-group ii. ii. The amine, carboxylic acid and R-group of an amino acid may be ionised under certain pH conditions. b) For amino acids (i– i), draw their most probable structure in biological fluid, pH 7.4. c) For amino acids (i– i), draw their most probable structure in stomach acid, pH 1. -arrow_forward
- Draw the structure of each peptide. Label the N-terminal and C-terminal amino acids and all amide bonds. a. Val-Glu b. Gly-His-Leu c. M-A-T-Tarrow_forwarda. Write the general structure of an amino acid. b. Identify what portion is common to all amino acids and what portion is different for each specific amino acid.arrow_forward2. Explain using two sentences the biological significance of the following peptides. A. Glucagon B. Insulin C. Glutathione D. Ceruloplasmin E. Oxytocinarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY