
Concept explainers
(a)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.47P
The product formed by the reaction of
Explanation of Solution
The given compound is,
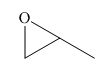
Figure 1
The nucleophile
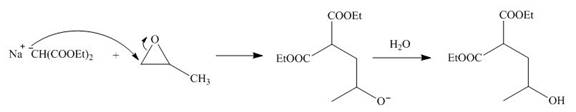
Figure 2
On the further treatment of
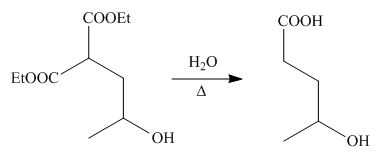
Figure 3
Hence, the product formed by the reaction of
The product formed by the reaction of
(b)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.47P
The product formed by the reaction of
Explanation of Solution
The given compound is,
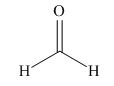
Figure 4
The nucleophile
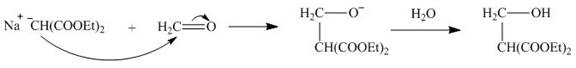
Figure 5
On the further treatment of
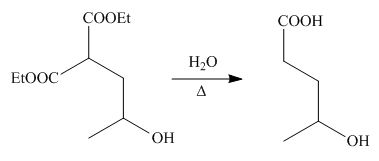
Figure 6
Hence, the product formed by the reaction of
The product formed by the reaction of
(c)
Interpretation: The products formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.47P
The product formed by the reaction of
Explanation of Solution
The given compound is,
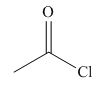
Figure 7
The nucleophile
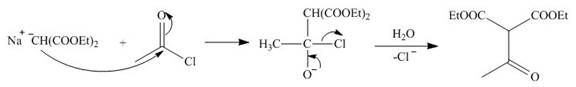
Figure 8
On the further treatment of
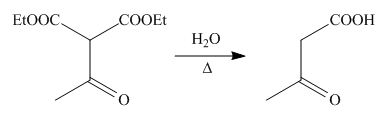
Figure 9
Hence, the product formed by the reaction of
The product formed by the reaction of
(d)
Interpretation: The product formed by the reaction of
Concept introduction: Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to the electrophilic site. The carbon atom in carbonyl compound acts as an electrophilic centre where a nucleophile attacks and gives an addition product.
Answer to Problem 23.47P
The products formed by the reaction of
Explanation of Solution
The given compound is,
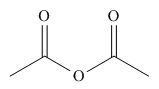
Figure 10
The nucleophile
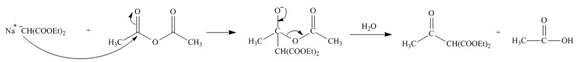
Figure 11
On the further treatment of
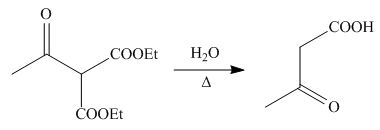
Figure 12
Hence, the products formed by the reaction of
The products formed by the reaction of
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- product formed by treating butanal with 1. AgNO3 in HH3 2. Acidified K2CrO4 3. LiAlH4arrow_forwardDraw the organic product(s) formed when CH3CH₂CH₂OH is treated with each reagent. a. H₂SO4 d. HBr g. TsCl, pyridine b. NaH h. [1] NaH; [2] CH₂CH₂Br e. SOCI₂, pyridine f. PBr3 c. HCI + ZnCl₂ Hint: NaH deprotonates the alcohol forming an alkoxidearrow_forwardBenzene undergoes electrophilic aromatic substitution reactions with aziridines in the presence of a Lewis acid such as AlCl3.a. What are the major and minor products of the following reaction?b. Would you expect epoxides to undergo similar reactions?arrow_forward
- Which set of reagents should be used to form the product shown below? O A. Heat, NaOH B. 1. HB(C6H11)2; 2. H202, NaOH O C. H20, H2S04, H9SO4 D. H20, H2SO4arrow_forwardWhich undergoes most rapid reaction on treatment with AICI3 and an acid halide a. b. C. d. benzene toluene chlorobenzene 1,4-dichlorobenzenearrow_forwardSynthesize each compound from benzene. Use a diazonium salt as one of the synthetic intermediates. COOH c. CH3- -CH,NH2 е. Но- -CH3 a. Br OH b. d. CH;CH2- -COOH -N=N- -NH2arrow_forward
- What is the reagent used for reaction 2? ... Reaction 1 Reaction 2 mСРВА ? A OH OH А) НЗРО4 B KMN04 c) H20 D NaOHarrow_forwardAn organic chemist reacts 1-butanol with SOCI2 in pyridine, removes the major organic product, and then reacts that product with KCN. What fınal major organic product does the chemist make? CN .CN CN A. C. D. Е. A E none of the above B.arrow_forwardDraw the product formed when pentanoic anhydride [(CH;CH,CH,CH,cO),0] is treated with each reagent. With some reagents, no reaction occurs. a. SOCI, b. H20 c. CH,OH d. NaCI e. (CH;CH),NH (excess) f. CH;CH,NH2 (excess)arrow_forward
- 5. What reagents are needed to convert toluene (C,H,CH,) to each compound? a. C.H.COOH b. C.H₂CH₂Br c. p-bromotoluene d. o-nitrotoluene e. p-ethyltoluene f.arrow_forwardconsider the followving reaction of a carbonyl to an alkene "CH2 P-Ph Ph Ph CH2 Phosphonium ylide What is the name of the reaction that converts carbonyls to alkenes? a. Witten Reaction b. Wittig Reaction c. Welker Reaction d. Whinging Reaction What steps are needed to generate the phosphonium ylide? a. Triphenylphosphine + CH,I – Treat with NaH b. Treat with NaH – Triphenylphosphine + CH;I c. Triphenylphosphine + CH, - Treat with NaOH d. Treat with NaOH – Triphenylphosphine + CH,arrow_forwardHow Wittig reagents are synthesized by a two-step procedure ?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





