
(a)
Interpretation: The
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
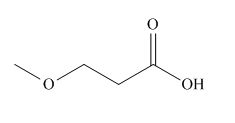
Figure 1
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
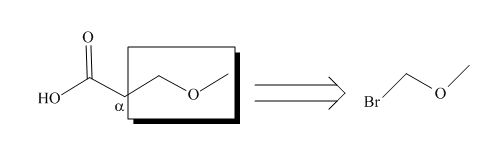
Figure 2
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
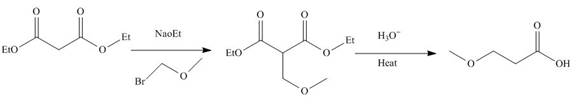
Figure 3
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate ion reacts with
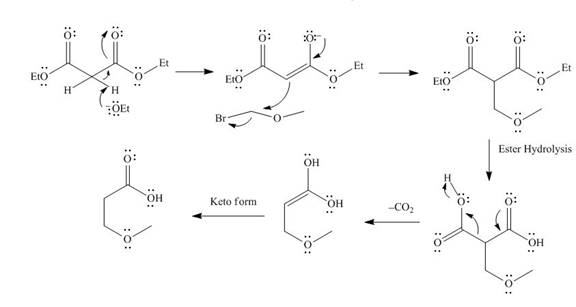
Figure 4
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
(b)
Interpretation: The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is to be predicted.
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
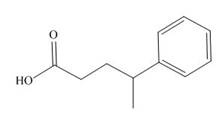
Figure 5
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
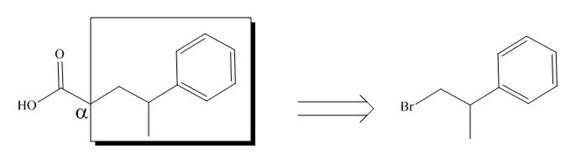
Figure 6
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
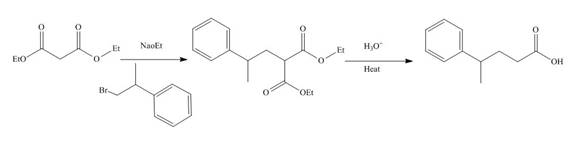
Figure 7
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate reacts with
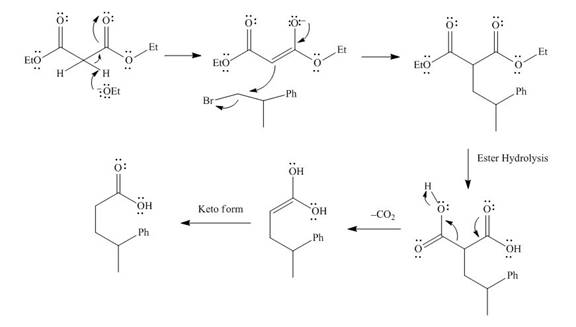
Figure 8
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
(c)
Interpretation: The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is to be predicted.
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
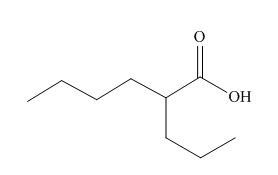
Figure 9
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
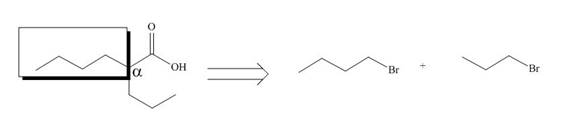
Figure 10
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
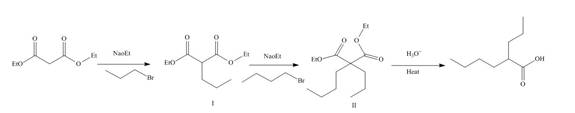
Figure 11
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate reacts with
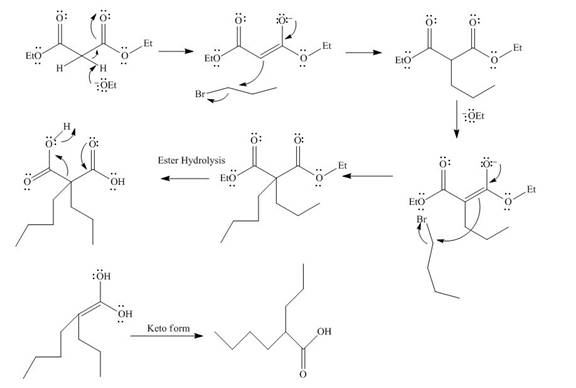
Figure 12
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Select the carbonyl compound which can form only a single enol via tautomerization. A. B. H C. D.arrow_forwardDraw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form asubstituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that canbe used as a synthetic detergent to clean away dirt.arrow_forwardDraw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form a substituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that can be used as a synthetic detergent to clean away dirtarrow_forward
- Which compound in each pair is the stronger acid? a. or b. orarrow_forwardElectron inductive effect of alkyl groups decreases the acidity in: A. acetic acid B. benzoic acid C. hydrochloric acid D nitric acidarrow_forwardProblem 16.2: Rank the following compounds by increasing heat of hydrogenation (1-lowest and 3-highest) a. b. C.arrow_forward
- d) Explain why A is less reactive than B towards a nucleophilic attack. H. A Barrow_forwardDraw a stepwise mechanism for the attached reaction that forms ether D. D can be converted to the antidepressant fluoxetine (trade name Prozac) in a single steparrow_forwardDraw a stepwise mechanism for the attached reaction, which involves two Friedel–Crafts reactions. B was an intermediate in the synthesis of the antidepressant sertralinearrow_forward
- Electrophilic Addition Soubong neblA-aleid rose 9160910 31 babeen ene singo 14.43 Draw the products formed when each compound is treated with one equivalent of HBr. a. b. C.arrow_forwardProblem 25.14 What nitro compound, nitrile, and amide are reduced to each compound? a. CH,CHCH,NH, CH,NH, b. Problem 25.15 What amine is formed by reduction of each amide? CONH, b. "NHCH Problem 25.16 Explain why isopropylamine [(CH).CHNH] can be prepared by reduction of a nitro compound, but cannot be prepared by reduction of a nitrile, even though it is a 1* amine. Problem 25.17 Which amines cannot be prepared by reduction of an amide? NH O O Darrow_forwardthe carbonyl donor or precursor in the following reaction is: a. A b. B c. C d. NaOHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
