
Concept explainers
(a)
Interpretation:
Procaine was one of the first local anesthetics. Its hydrochloride salt is marketed as Novocain.
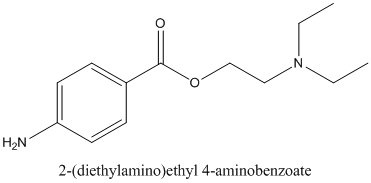
If procaine is chiral or not and if it contains any stereocenter should be identified.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
(b)
Interpretation:
The nitrogen atom of procaine which is a stronger base should be identified.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
(c)
Interpretation:
A structural formula for the salt formed by treating procaine with one mole of HCl, showing which nitrogen is protonated and bears the positive charge should be determined.
Concept Introduction:
Procaine contains amino ester group, it is a local anesthetic drug. It is required to relief the pain caused by injection of penicillin. It is also required in dentistry. In some regions, procaine is popular as Novocain.
Trending nowThis is a popular solution!

Chapter 15 Solutions
Introduction To General, Organic, And Biochemistry
- What is the pH, along with the concentration of each form of serine, in a 0.020 M solution of serine?arrow_forwardThe pKa of ascorbic acid (vitamin C, page 55) is 4.17, showing that it is slightly more acidic than acetic acid (CH3COOH, pKa 4.74). Compare the most stable conjugate base of ascorbic acid with the conjugate base of acetic acid, and suggest why these two compounds have similar acidities, even though ascorbic acid lacks the carboxylic acid (COOH) group.arrow_forwardName two techniques that can be employed to extract oil from seeds Hence,Name four processes that may be used to refine oils,What is the description for the term rancidity of oils? ,Name two causes of rancidity in oils, What is acid value of oils? Therefore How many moles of KOH is required per mole of fat in saponification reactions?arrow_forward
- Write a net ionic equation to show that diethylamine, (C2H3)2NH, behaves as a Bronsted-Lowry base in water. BL base BL acid BL acid BL base H20 +arrow_forwardFill in the left side of this equilibrium constant equation for the reaction of diethylmethylamine (C,H13N), a weak base, with water. Ü - K,arrow_forwardWrite a net ionic equation to show that piperidine, C5H11N, behaves as a Bronsted-Lowry base in water. BL base BL acid BL acid BL base + H2O +arrow_forward
- Complete the equation to show how pyridine, C5H5N, acts as a Brønsted-Lowry base in water. equation: C5H5N + H2Oarrow_forwardWrite the chemical equation for the acid dissociation of acetaminophen, C8H9O2N. Write the Ka expression for the acid dissociation of acetaminophen.arrow_forward3) Calculate the pH of a 27 mg/L solution of hydrocyanic acid (HCN). The pKa of hydrocyanic acid is 9.3.arrow_forward
- You are going to be using Isoleucine in a buffer. The pKa of the carboxylate group of Isoleucine is 2.36 If you have a 0.1 M solution of Isoleucine at pH 3.22, what fraction (or percent) of the solution is in the deprotonated (COO- ) form?arrow_forwardCalculate the pH of a 0.100 M alanine (HL/intermediate form) solution. pKb1 = 4.29 and pKb2 = 11.67.arrow_forwardWhy is H2SeO4 a weak acidarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

