
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 11.12P
Each compound given in this problem is a common organic solvent. From each pair of compounds, select the solvent with the greater solubility in water.
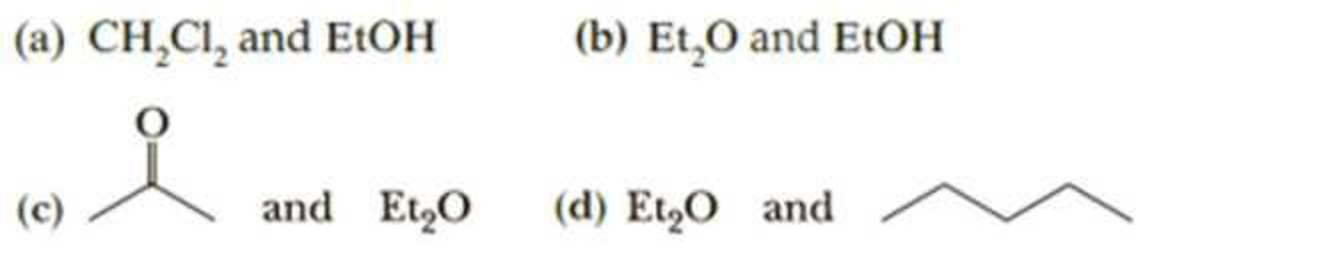
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Choose the best explanation for why these
compounds are all less than fifteen carbons.
A) Aldehydes with longer alkyl chains
become increasingly nonpolar and the LDF
are stronger than hydrogen bonding with
ethanol.
B) Ethanol molecules will hydrogen bond
with the oxygen of the aldehydes. However,
longer alkyl chains interrupt the ability of
the aldehyde to hydrogen bond with
ethanol which decreases the solubility.
C) Ethanol molecules will form strong LDF
interactions between the alkyl chains of the
aldehydes and the alkyl groups of ethanol.
The strong attractions cause the aldehydes
to precipitate out of solution.
D) When aldehydes chain length increases,
the aldehydes have stronger intermolecular
force with the small ethanol solvent
molecules than the ethanol-ethanol
intermolecular force.
Organic Chemistry
Incorrect Separation Scheme
Cannot be hand-drawn
*see attached for the incorrect separation scheme provided.
The top of the separation scheme shows what other compound is mixed with your molecule (2,6-dimethyloct-2-ene). Assume for the purposes of this assignment that both compounds are solid at room temperature. Also assume that both compounds are soluble in ether, except ionic compounds. The goal of the separation is the isolate each of the two compounds from the mixture.
Below the incorrect separation scheme write a discussion of this incorrect scheme identifying all of the mistakes in the separation scheme. Keep in mind that there will be more than one mistake in the scheme.
For each mistake, give a detailed, scientific explanation of why it is incorrect.
Rank the following molecules in increasing order of water solubility and boiling point.
Chapter 11 Solutions
Organic Chemistry
Ch. 11.2 - Write IUPAC and common names for these ethers. (a)...Ch. 11.3 - Arrange these compounds in order of increasing...Ch. 11.4 - Show how you might use the Williamson ether...Ch. 11.4 - Show how ethyl hexyl ether might be prepared by a...Ch. 11.5 - Account for the fact that treatment of tert-butyl...Ch. 11.5 - Draw structural formulas for the major products of...Ch. 11.6 - Prob. 11.7PCh. 11.8 - Draw the expected products of Sharpless...Ch. 11.9 - Prob. AQCh. 11.9 - Prob. BQ
Ch. 11.9 - Prob. CQCh. 11.9 - Prob. DQCh. 11 - Write names for these compounds. Where possible,...Ch. 11 - Prob. 11.11PCh. 11 - Each compound given in this problem is a common...Ch. 11 - Account for the fact that tetrahydrofuran (THF) is...Ch. 11 - Prob. 11.14PCh. 11 - Write equations to show a combination of reactants...Ch. 11 - Propose a mechanism for this reaction.Ch. 11 - Prob. 11.17PCh. 11 - Prob. 11.18PCh. 11 - Prob. 11.19PCh. 11 - Prob. 11.20PCh. 11 - Ethylene oxide is the starting material for the...Ch. 11 - Prob. 11.22PCh. 11 - Predict the structural formula of the major...Ch. 11 - The following equation shows the reaction of...Ch. 11 - Propose a mechanism to account for this...Ch. 11 - Acid-catalyzed hydrolysis of the following epoxide...Ch. 11 - Prob. 11.27PCh. 11 - Prob. 11.28PCh. 11 - Prob. 11.29PCh. 11 - Propose a mechanism for the following...Ch. 11 - Show reagents and experimental conditions to...Ch. 11 - Starting with cis-3-hexene, show how to prepare...Ch. 11 - Show reagents to convert cycloheptene to each of...Ch. 11 - Show reagents to convert bromocyclopentane to each...Ch. 11 - Prob. 11.35PCh. 11 - Starting with acetylene and ethylene oxide as the...Ch. 11 - Following are the steps in the industrial...Ch. 11 - Prob. 11.38PCh. 11 - Prob. 11.39PCh. 11 - Aldehydes and ketones react with one molecule of...Ch. 11 - Prob. 11.42PCh. 11 - Write the products of the following sequences of...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - Using your reaction roadmap as a guide, show how...Ch. 11 - During the synthesis of the antiasthmatic drug...Ch. 11 - Prob. 11.48P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. What letter has an aldehyde with molecular formula C₄H₈O. 2. What letter has an ester with molecular formula C₄H₈O₂ 3. What letter has a ketone with molecular formula C₄H₈O₂ 4. What letter has a carboxylic acid with molecular formula C₄H₈O₂arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l)CH3CH2CH2CO2H(l)+CH2CH3OH(l)⟶H+CH3CH2CH2CO2CH2CH3(l)+H2O(l) A chemist ran the reaction and obtained 5.40 g of ethyl butyrate. What was the percent yield, The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.45g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Part A Given 7.30 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. Part B A chemist ran the reaction and obtained 5.95 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. Part C The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 7.30 gg of…arrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring. It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.45 gg of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%% yield? Express your answer in grams to three significant figures. A chemist ran the reaction and obtained 5.50 gg of ethyl butyrate. What was the percent yield? Express your answer as a percent to three significant figures. The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0%% yield. How many grams would be produced from 8.45 gg of butanoic acid and excess…arrow_forwardAcid catalyzed dehydration reaction of 2-methyl-1-butanol produces 2-methyl-2-butene as the major product. Also acid catalyzed dehydration reaction of 3-methyl-1-butanol give the same product as major product. Explain the reason why both of the reaction produce the same product as the major product.arrow_forwardRank the following in order of decreasing solubility in benzene. gallic acid ethyl benzene resorcinolarrow_forward
- Provide the necessary reagents to accomplish the desired organic reactions. Please number the steps and note that some reactions will require more than one step.arrow_forwardWhy is it important that we are very careful in how much acid is added to neutralize the reaction? (Only pick one answer) If the solution becomes very acid it can be harmful to the skin Too much acid will increase vanillyl alcohols water solubility and inhibit crystal formation Too much acid will decrease vanillyl alcohols water solubility and inhibit crystal formation Excess acid will deprotonate the vanillyl alcohol and inhibit crystal formationarrow_forwardComplete the following transformationsarrow_forward
- Organic Chemistry Acid catalyzed dehydration reaction of 2-methyl-1-butanol produces 2-methyl-2-butene as the major product. Also acid catalyzed dehydration reaction of 3-methyl-1-butanol give the same product as major product. Explain the reason why both of the reaction produce the same product as the major product.arrow_forwardFor each of the following, draw a structural formula that meets the stated requirements, then name each structureyour drew (unless otherwise indicated) according to either IUPAC or common nomenclature.arrow_forwardDraw the structure of the major organic product(s) for the following reaction between an acetylenic anion and an alkyl halide. (The reaction stoichiometry is 1:1.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY