
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.32P
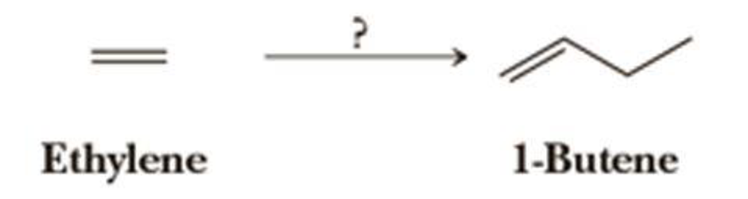
Using your reaction roadmap as a guide, show how to convert ethylene into 1-butene. All of the carbon atoms of the target molecule must be derived from ethylene. Show all intermediate molecules synthesized along the way.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
2-chloropropane is a major product of the reaction of chlorine with propane under ultraviolet light. Write the mechanism for this reaction including the initiation step and the two propagation steps.
Convert the alcohol, n-propanol, to n-propyl fluoride in 2 steps. List the reagents in the order you would use them.
HO-A-OH
HO-C-B-C-OH
ethylene glycol
(A group)
terephthalic acid
(B group)
The starting materials fer this reaction are ethylene glycol and terephthalic acid. The letters A and B represent organic groups that are unreactive.
What reactive organic functional group does ethylene glycol (containing the A functional group) contain? Enter its name.
Recheck
Next
(1 of 7)
2nd attempt
Chapter 7 Solutions
Organic Chemistry
Ch. 7.2 - Write the IUPAC name of each compound.Ch. 7.2 - Write the common name of each alkyne.Ch. 7.5 - Prob. 7.3PCh. 7.7 - Draw a structural formula for a hydrocarbon with...Ch. 7.7 - Hydration of 2-pentyne gives a mixture of two...Ch. 7.9 - Prob. 7.6PCh. 7 - Prob. 7.7PCh. 7 - Show how to prepare each alkyne from the given...Ch. 7 - Prob. 7.9PCh. 7 - Complete each acid-base reaction and predict...
Ch. 7 - Draw structural formulas for the major product(s)...Ch. 7 - Draw the structural formula of the enol formed in...Ch. 7 - Prob. 7.13PCh. 7 - Prob. 7.14PCh. 7 - Prob. 7.15PCh. 7 - Show reagents and experimental conditions you...Ch. 7 - Show reagents and experimental conditions you...Ch. 7 - Show how to convert 1-butyne to each of these...Ch. 7 - Prob. 7.19PCh. 7 - Show reagents and experimental conditions to bring...Ch. 7 - Show reagents to bring about each conversion.Ch. 7 - Propose a synthesis for (Z)-9-tricosene...Ch. 7 - Propose a synthesis of each compound starting from...Ch. 7 - Show how to prepare each compound from 1-heptene....Ch. 7 - Prob. 7.25PCh. 7 - Prob. 7.26PCh. 7 - Following is the structural formula of the...Ch. 7 - The standard procedure for synthesizing a compound...Ch. 7 - Prob. 7.29PCh. 7 - Prob. 7.30PCh. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Using your reaction roadmap as a guide, show how...Ch. 7 - Prob. 7.35P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 12-50 Draw the structural formula of an alkene that undergoes acid-catalyzed hydration to give the indicated alcohol as the major product. More than one alkene may give each alcohol as the major product. 3-Hexanol 1-Methylcyclobutanol 2-Methyl-2-butanol 2-Propanolarrow_forwardShow how to convert ethylene to these compounds. (a) Ethane (b) Ethanol (c) Bromoethane (d) 2-Chloroethanol (e) 1,2-Dibromoethane (f) 1,2-Ethanediol (g) Chloroethanearrow_forward10-9 Is there any difference between vanillin made synthetically and vanillin extracted from vanilla beans?arrow_forward
- Write down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH,—O—C—(CH2)—CH=CH-CH,—CH=CH—(CH2) — CH3 CH-0- -(CH2)–CH=CH(CH2)CH3 O CH,—O-C=(CH2)=CH=CH(CH2)—CH3 Separate each name with a comma and a space. You will find useful information in the ALEKS Data resource. type your answer...arrow_forward10) Synthesis: Make the following products from a suitable cyclic alkene starting material. Look at the functional group PATTERN present in the molecule, including stereochemistry. ♡ Br Brarrow_forwardI am completing my lab assignment called synthesis of an alcohol by hydrating an alkene. Get two small test tubes. Place 1 mL of bromine water into each of the two test tubes.Add about three drops of the product and hex-1-eneinto each of the two test tubes. Swirl the contents of the tubes. Record you observations. My observations were: cyclohexene turned clear, the cyclohexanol turned yellow. Based on these reults, was I able to synthesize the of hexan-2-ol from the hydration of hex-1-ene?arrow_forward
- What is the slow, rate-determining step, in the acid-catalyzed dehydration of 2- butanol? Loss of a b-hydrogen from the carbocation to form an alkene. Protonation of the alcohol to form an oxonium ion. Loss of water from the oxonium ion to form a carbocation. The simultaneous loss of a B-hydrogen and water from the oxonium ion.arrow_forwardStarting with 2-propanol, write out a series of chemical reactions that would allow you to produce the following molecule: H3C- CH3 CH-CH3 CH3 CH T CH3 CH3arrow_forward2. Using structural diagrams to explain the synthesis of butyl ethanoate from an alkane and an alcohol. Make sure you have the names of the substances and the reaction conditions of the reactions of the steps that consists your synthesis HC-C H- styrene HH ethane-1,2-diol OH НО. JOL -0-H benzene-1.4-dicarboxylic acid.arrow_forward
- Cellosolve® is the trade name for 2-ethoxyethanol, a common industrial solvent. Thiscompound is produced in chemical plants that use ethylene as their only organic feedstock. Show how you would accomplish this industrial process.arrow_forwardWrite down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH2−O−C—(CH2);–CH=CH–CH2–CH=CH—(CH2)4—CH3 CH-O-C-(CH2)14-CH3 O 11 CH2−O−C— (CH2)14 — CH3 Separate each name with a comma. You will find useful information in the ALEKS Data resource. 1 010 Continue O a X 000 Y F8 F9 Submiarrow_forwardMixing cyclohexanol with phosphoric acid is an exothermic process, whereas the production of cyclohexene is endothermic. Construct an energy diagram showing the course of this reaction. Label the diagram with the starting alcohol, the oxonium ion (the protonated alcohol), the carbocation, and the product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY