
Concept explainers
Interpretation:
The IUPAC names of each of the given compounds are to be given by using
Concept introduction:
The priority of groups in nomenclature is assigned according to Cahn-Ingold-Prelog (CIP Rule) convention rules:
The higher the
If priority cannot be assigned according to atomic mass, then assign the priority according to first point of difference.
If both the priority groups are on the same side of double-bonded carbon atom, then it is known as
But, if both the priority groups are diagonal to each other, then it is
Rule for R/S Nomenclature:
4B(+)3B(-)2B(+)1B(-)
4B(+) means if 4th priority group is below then from 1 to 2 to 3 if leads a clockwise rotation then its R and if anticlockwise then it is S.
3B(-) means if 3rd priority group is below then from 1 to 2 to 4 if leads a clockwise rotation then its S and if anticlockwise then it is R.
Answer to Problem 1PP
Solution:
Explanation of Solution
a)
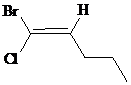
The IUPAC name with
Assign the priority to four groups, –Br gets first priority, pentyl group gets second, –Cl gets third, and –H gets fourth. Since both the priority groups are diagonal to each other, it is
Select the longest carbon chain. The parent hydrocarbon chain is pentane and hence pent- will be used and double bond is at the second position. Therefore, pent-1-ene is used.
Chloro and bromo groups are at first position. So, 1-bromo-1-chloropent-1-ene will be used.
Hence, the IUPAC name of the compound will be
b)
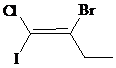
The IUPAC name with
Assign the priority to four groups, –I gets first priority, –Br gets second, –Cl gets third, and ethyl group gets fourth. Since both the priority groups are diagonal to each other, it is
Select the longest carbon chain. The parent hydrocarbon chain is butyl and hence but- will be used and double bond is at the first position. Therefore, but-1-ene is used.
Chloro and iodo groups are at first position and bromo group is at second position. So, 2-bromo-1-chloro-1-iodobut-1-ene will be used.
Hence, the IUPAC name of the compound will be
c)
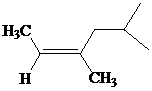
The IUPAC name with
Assign the priority to four groups, 2-methylpropyl gets first priority, both methyl groups get second, and –H gets third. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the second position. Therefore, hex-2-ene is used.
There are two methyl groups at third and fifth positions. So, 3,5-dimethylhex-2-ene will be used.
Hence, the IUPAC name of the compound will be
d)
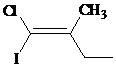
The IUPAC name with
Assign the priority to four groups, –I gets first priority, –Cl gets second, ethyl group gets third, and methyl group gets fourth. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is butyl and hence but- will be used and double bond is at the first position. Therefore, but-1-ene is used.
There is one methyl group at second position, and chloro and iodo groups at first position. So, 1-chloro-1-iodo-2 methylbut-1-ene will be used.
Hence, the IUPAC name of the compound will be
e)
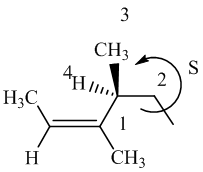
The IUPAC name with
Assign the priority to four groups, 1-methylpropyl gets first priority, both methyl groups get second, and –H gets third. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the second position. Therefore, hex-2-ene is used.
There are two methyl groups at third and fourth position. So, 3,4-dimethylhex-2-ene will be used.
Carbon-4 is a chiral carbon. Assigning priority to the groups, 2-butene gets first priority, ethyl gets second, methyl gets third, and hydrogen gets fourth.
Move 1-2-3, it is in anticlockwise direction. So, it is
Hence, the IUPAC name of the compound will be
f)
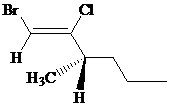
The IUPAC name can be done using the following steps:
Assign the priority to four groups, –Br gets first priority, –Cl gets second, 1-methylbutyl gets third, and –H gets fourth. Since both the priority groups are on the same side, it is
Select the longest carbon chain. The parent hydrocarbon chain is hexyl and hence hex- will be used and double bond is at the first position. Therefore, hex-1-ene is used.
There is one methyl group at third position, and chloro at second and bromo at first position. So, 1-bromo-2-chloro-3-methylhex-1-ene will be used.
Carbon-3 is a chiral carbon. Assigning priority to the groups, 1-bromo-2-chloroethene gets first priority, propyl gets second, methyl gets third, and hydrogen gets fourth.
Rotate the molecule such that –H is at a horizontal position and then move 1-2-3, it is in anticlockwise direction. So, it is
Hence, the IUPAC name of the compound will be
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
- 5.21 Give IUPAC names for the following substances (red = 0, blue = N). (a) (b)arrow_forwardGive IUPAC names for the following structures. (If appropriate, specify relative stereochemistry.) (a) (b) S Sarrow_forward5.34 Select the compound in each of the following pairs that will be converted to the corresponding alkyl bromide more rapidly on being treated with hydrogen bromide. Explain the reason for your choice. (a) 1-Butanol or 2-butanol (b) 2-Methyl-1-butanol or 2-butanol (c) 2-Methyl-2-butanol or 2-butanol (d) 2-Methylbutane or 2-butanol dovolonentanol or cyclohexanolarrow_forward
- 5.20 Name each of the following compounds according to substitutive IUPAC nomenclature: (a) (CH3),CHCH2CH2CH2B1 (b) (CH3)2 CHCH2CH2 CH2OH (c) Cl3 CCH2B C12CHCHB1 (d) Cl (e) CF3CH2OH (f) ОН CH3 (g) ОН CH3 (h) Br (i) ОНarrow_forwardO PRACTICE PROBLEM 8.14 Starting with any needed alkene (or cycloalkene) and assuming you have deuterioace- tic acid (CH3CO,D) available, outline syntheses of the following deuterium-labeled compounds.s el en olad lo nohibbs ad CH3 (a) (CH3)2CHCH2CH,D (b) (CH3),CHCHDCH3 (c) (+ enantiomer) (d) Assuming you also have available BD3:THF and CH3CO2T, can you suggest a synthesis of the following? hab erl (+ enantiomer)he imo (nwond-ben) CH3 H. (asoholea)arrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctanearrow_forward
- Provide three-dimensional structures for the missing boxed structures and formulas for missing reagents. (S)-A (C5H₁ Br) Nat (S)-B (C₂H₁2) (S)-C (C₂H16)arrow_forward7.55 Acid-catalyzed dehydration of 2,2-dimethyl-1-hexanol gave a number of isomeric alkenes including 2- methyl-2-heptene as shown in the following equation. H2SO4 НО heat (a) Write a stepwise mechanism for the formation of 2-methyl-2-heptene, using curved arrows to show the flow of electrons. (b) What other alkenes do you think are formed in this reaction?arrow_forward8. (a) Benzene derivatives exhibit medium to strong absorption in UV-region. Explain why aniline and phenoxide ion have strong UV-absorptions.arrow_forward
- Answer ALL parts of this question. The structure shown below is that of the E-isomer of tripolidine, an antihistamine (the E-isomer is more active than the Z-isomer in this capacity): (a) (b) H3C- oto Explain how the Cahn Ingold Prelog sequence rules can be used to rank groups in order of priority, using the four groups attached to the C=C double bond in tripolidine as illustrations. Based on the order of priority of the four groups determined in part (a) of this question, explain why the isomer of tripolidine shown above is defined as the E-isomer. (c) In general terms, explain why this isomer might have different effects in the human body from its geometric isomer.arrow_forward4.33 Select the compound in each of the following pairs that will be converted to the corresponding alkyl bromide more rapidly on being treated with hydrogen bromide. Explain the reason for your choice. (a) 1-Butanol or 2-Butanol (b) 2-Methyl-1-butanol or 2-butanol (c) 2-Methyl-2-butanol or 2-butanol (d) 2-Methylbutane or 2-butanol (e) 1-Methylcyclopentanol or cyclohexanol Draw the energy diagrams of an SN1 reaction and an SN2 reaction. Include in your drawing anexample reaction. Identify the rate limiting step and label it as unimolecular or bimolecular.arrow_forwardPractice Problem 7.16 When the compound called isoborneol is heated with 9 M sulfuric acid, the product of the reaction is the compound called camphene and not bornylene, as one might expect. Using models to assist you, write a step-by-step mechanism showing how camphene is formed. HO H,O not heat Isoborneol Camphene Bornylenearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





