
Concept explainers
Predict all approximate bond angles about each highlighted carbon atom. To make these predictions, use valence-shell electron-pair repulsion (VSEPR) theory (Section 1.4).
(a) 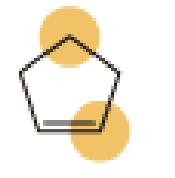
(b) 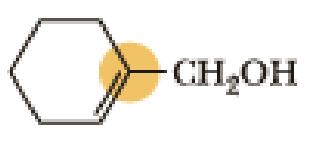
(c) 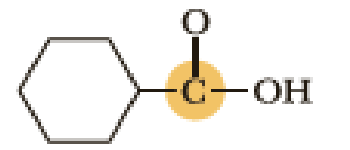
(d) 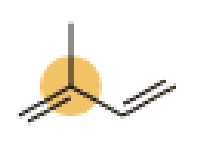
(e) 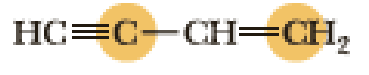
(a)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
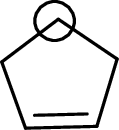
The marked carbon atom has four bond pairs. Thus it has four electron domains and is in tetrahedral geometry. Hence, the bond angle is
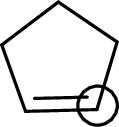
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
(b)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
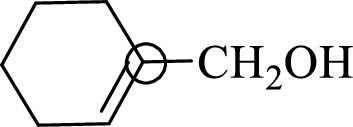
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
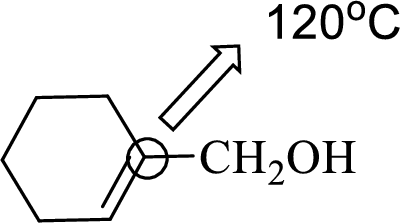
(c)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
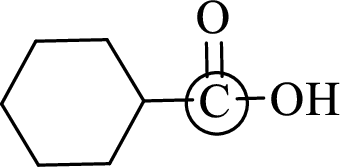
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
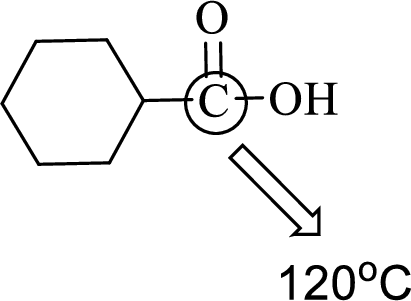
(d)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
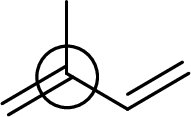
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
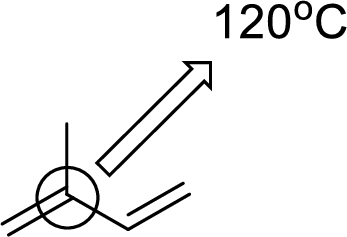
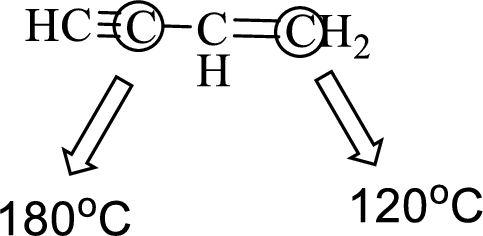
(e)
Interpretation:
An approximate bond angle of the highlighted carbon atom has to be predicted using VSEPR theory.
Concept Introduction:
VSEPR Theory:
The basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then tend to be in position in order to minimize the repulsions.
According to VSEPR theory, the geometry is predicted by the minimizing the repulsions between electron-pairs in the bonds and lone-pairs of electrons. The VSEPR theory is summarized in the given table as,
- • Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
- • Electro-domain geometry includes both bond pairs and lone pairs of central atom for determining the geometry of molecule.
Explanation of Solution
Bond angle can be determined based on the electron-domain geometry.
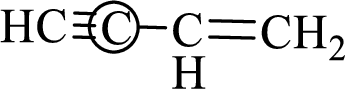
The marked carbon atom has two bond pairs. Thus it has two electron domains and is in linear geometry. Hence, the bond angle is
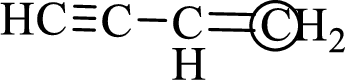
The marked carbon atom has three bond pairs. Thus it has three electron domains and is in trigonal planar geometry. Hence, the bond angle is
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
- 1. Draw the bond-line structures for the below compounds. (a) (CH3)3C(CH2)2OH (b) CH3CH₂CH(CH3)CH₂CH₂COOHarrow_forwardDraw structural formulas of at least two alkynes of each molecular formula.(1) C6H10 (2) C8H12 (3) C7H8arrow_forwardWrite the IUPAC name for each of the following molecules. (a) (b) (c) (d) Br Br CI Br F Br (e) (f) (g) (h) (1) Farrow_forward
- Which of these is not aromatic? (A) (B) (C) (D) CH CHarrow_forward2. a) Draw the structures of Perylene dianhydride, Naphthalene and Salicylic acid b) Arrange the compounds in order of increasing polarity considering the structures and Rf valuesarrow_forward1. Write bond-line formulas for (a.) four aldehydes with the formula of C5H10O (b) three ketones that have the formula C5H10O (c.) four carboxylic acids with the formula C5H10O2 (d.) three esters with the formula C5H10O2arrow_forward
- The skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forwardFor each pair of compounds, predict which one has the higher molecular dipole moment, and explain your reasoning.(a) 1-bromopropane or cyclopropanearrow_forwardFor each of the following compounds, identify all groups that would be considered substituents and then indicate the systematic name for each compound. (a) (b) (d) (e)arrow_forward
- Structures with the molecular formula C7H12O2 that contain two units of unsaturation. Create a single structural formula for each isomer that fits the description given in the boxes:A) C7H12O2 isomer containing a carbon-carbon ringB) C7H12O2 isomer that is a KetoneC) C7H12O2 isomer that is a Carboxylic AcidD) C7H12O2 isomer containing sp3 hybridized carbons bound to each otherE) C7H12O2 ester which contains a carbon-carbon ringarrow_forwardExamine the ungraded ball-and-stick model to determine the three-dimensional structure of the molecule. On the corresponding 2D structure, draw one wedge bond and one dash bond over two existing bonds to indicate the same arrangement of atoms in space. The narrow part of each wedge-and-dash bond should be towards the same central carbon atom. Click on the three-dimensional molecular structure of (S)-1-chloro-2,3-dimethylbutane and drag to rotate it, or use the controls provided. 80 F3 $ 4 R F 000 COD F4 D V % 5 C T G MA F5 B OH 6 CL MacBook Pro Y Fo H & 7 N da F7 U * 00 8 M DII FO - Change two bonds to stereobonds. K Select Draw Rings More ////// 3 ( 9 H CH₂ */ CH₂ G DD F9 H O V H,C ) O command F10 P A C - H F11 Q2Q Cl { option + = F12 Erase = ] (1) delearrow_forwardBromine is a larger atom than chlorine, but the equilibrium constants in Table 3.9 indicate that a chloro substituent has a greater preference for the equatorial position than does a bromo substituent. Suggest an explanation for this fact.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





