
Draw the structure corresponding to each IUPAC name.
a.
b.
c.
d. cyclobutylcycloheptane
e.
f.
g
h.
i.
j.
(a)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.40P
The structure corresponding to
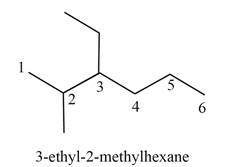
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
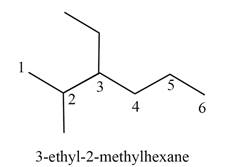
Figure 1
The structure corresponding to the given name is shown in Figure 1.
(b)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
4. First identify the word root for the given compound.
5. The suffix used in the compound like –ene.
6. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.40P
The structure corresponding to
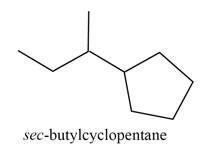
Explanation of Solution
The given IUPAC name is
The given name is

Figure 2
The structure corresponding to the given name is shown in Figure 2.
(c)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
1. First identify the word root for the given compound.
2. The suffix used in the compound like –ene.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.40P
The structure corresponding to
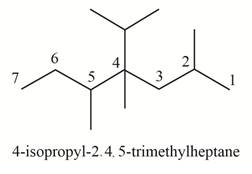
Explanation of Solution
The given IUPAC name is
The given name is
Thus, the correct structure of

Figure 3
The structure corresponding to the given name is shown in Figure 3.
(d)
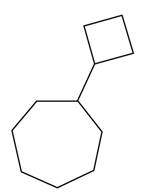
Interpretation: The structure corresponding to cyclobutylcycloheptane is to be drawn.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.40P
The structure corresponding to cyclobutylcycloheptane is shown as,
Explanation of Solution
The given IUPAC name is cyclobutylcycloheptane. The given name is cyclobutylcycloheptane. The word root used in this is hept. It means structure contains seven carbon atoms. The hydrocarbons that are attached to the longest chain are called substituents and they are written as prefix in alphabetical order. Therefore, the name suggests that one cyclobutyl substituents is attached to the carbon atom of cycloheptane.
Thus, the correct structure of cyclobutylcycloheptane is shown below.
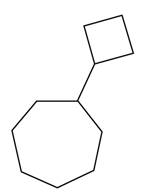
Figure 4
The structure corresponding to the given name is shown in Figure 4.
(e)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.40P
The structure corresponding to
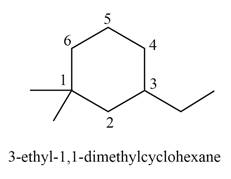
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 5
The structure corresponding to the given name is shown in Figure 5.
(f)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.40P
The structure corresponding to
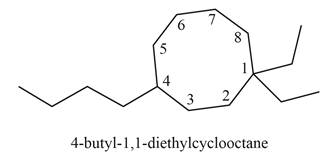
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 6
The structure corresponding to the given name is shown in Figure 6.
(g)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.40P
The structure corresponding to
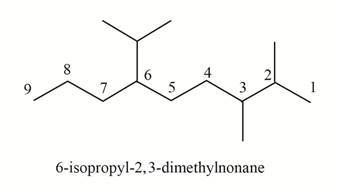
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 7
The structure corresponding to the given name is shown in Figure 7.
(h)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Answer to Problem 4.40P
The structure corresponding to
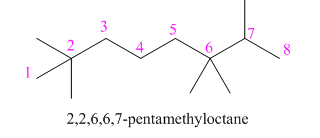
Explanation of Solution
The given IUPAC name is
Thus, the correct structure of

Figure 8
The structure corresponding to the given name is shown in Figure 8.
(i)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
4. First identify the word root for the given compound.
5. The suffix used in the compound like –ene.
6. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.40P
The structure corresponding to
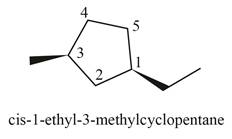
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
7. First identify the word root for the given compound.
8. The suffix used in the compound like –ene.
9. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of
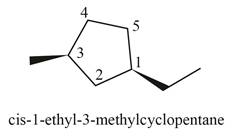
Figure 9
The structure corresponding to the given name is shown in Figure 9.
(j)
Interpretation: The structure corresponding to
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing structural formula from IUPAC are:
10. First identify the word root for the given compound.
11. The suffix used in the compound like –ene.
12. Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 4.40P
The structure corresponding to
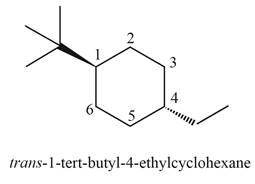
Explanation of Solution
The given IUPAC name is
Rules for writing structural formula from IUPAC are
13. First identify the word root for the given compound.
14. The suffix used in the compound like –ene.
15. Identify the position, location, and number of the substituent bonded to the carbon chain.
The given name is
Thus, the correct structure of

Figure 10
The structure corresponding to the given name is shown in Figure 10.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Thermodynamics, Statistical Thermodynamics, & Kinetics
Fundamentals of Heat and Mass Transfer
Chemistry: Structure and Properties (2nd Edition)
Chemistry: Atoms First
Chemistry
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- CH;CH-CH, 12. CH3-CH-CH-CH; CH-CH; What is the correct IUPAC name for the compound above? a. 2,3,4-trimethylhexane b. 2-ethyl-3-isopropylbutane c. 1,2,3,4-tetramethylpentane d. 3,4-dimethylheptanearrow_forward8. Name each alkyne. A. CH3CH2CH2C=CH B. CH3CH2CH2C=CCH3arrow_forwardCH, 20. сH,-CH-CН CH,CH, What is the correct IUPAC name for the compound above? O a. 3-ethyl 3- methyl-1-propene b. 3-methyl-1-pentene c. sec-butylethene d. 1-isohexenearrow_forward
- Draw a complete structure for each alkene or alkyne. a. CH2 CHCH2CH3 b. CH3C CCH2CH3arrow_forwardCH-CH3 CH3-CH2-CH2-CH-CH-CH3 CH2 ČH-CH3 What is the IUPAC name of this structure? OA. 2-methyl-3-propyloctane OB. 2-ethyl-3-propylhexane OC.3-ethyl-4-propylhexane OD.3-methyl-4-propylheptanearrow_forwardGive the structure corresponding to each IUPAC name. a.1,2-dimethylcyclobutane b.1,1,2-trimethylcyclopropane c. 4-ethyl-1,2-dimethylcyclohexane d.1-sec-butyl-3-isopropylcyclopentane e.1,1,2,3,4-pentamethylcycloheptanearrow_forward
- Name each alkane. a. CH3-CH-CH3 ČH; CH; CH3 b. CH3-CH-CH2-CH-CH2 ČH3 CH, CH, c. CH3-C-C-CH3 ČH, ČH3 CH3 CH, CH2 d. CH3-CH-CH2-CH-CH-CH2-CH2-CH3 ČH3arrow_forwardDraw the structure for each compound using wedges and dashed wedges.a. cis-1,2-dimethylcyclopropaneb. trans-1-ethyl-2-methylcyclopentanearrow_forward1. Which of the following is an ACYCLIC UNSATURATED hydrocarbon? * A. 2-Ethyl-1,1-dimethylcyclopentane B. 3-Ethyl-3-methylhexane C. 5-Methyl-1,3-hexadiyne D. 5-Ethenyl-1,3-cyclohexadiene 2. Which of the following is a CYCLIC UNSATURATED hydrocarbon? * A. 3-Ethenyl-3-methylcyclopropene B. 3-Ethyl-3-methylpentane C. Cyclopropylcyclohexane D. 2,2,7-Trimethyl-3-octyne 3. Refer to the FIGURE attached. Identify the name of family of the organic compound based on the functional group present in the structure. A. Ester B. Acid anhydride C. Ketone D. Aldehydearrow_forward
- 1. Draw the following molecules given their IUPAC name. a) 3-Methylheptane b) 3-isopropyl-2-methylhexane c) 2,2,4-trimethylpentane d) 2,2,7-trimetthyloctane e) 2-bromo-1,1-dimethylcyclohexane f) 2-methyl-1-ethyl-1,3-dipropylcyclopentane 2. Although a structure can be drawn for 2-methyl-2-isopropylheptane, the molecule is not named correctly using IUPAC rules. Draw the structure, and then give the appropriate IUPAC name for the molecule.arrow_forwardDraw a structure a. 3,4-dimethylpent-1-yneb.3-methyl-3-ethyl-1-butenec. 3,3-dimethyl-4-decened.1,1-dimethyl-4-ethyl-2,5-cyclohexadienee. 4-ethyl-2,3-dimethyl-2-heptenef. 1-chlorocyclopropeneg. 2,6-dimethyl-2,5-octadieneh. 1-cyclobutyl-3-methyl-1-butynei. 5-bromo-2-chlorotoluenearrow_forward1. It is an acyclic unsaturated hydrocarbon one or more carbon-carbon double bonds: A. Alkene B. Cycloalkene C. Alkyne D. Cycloalkyne 2. Which of the following compounds DOES NOT FOLLOW its IUPAC nomenclature system? * A. 3-Chloro-8-iodocyclooctene B. 3-Decyne C. 1-Chloro-1-methyl-2,4-heptadiene D. 3,3-Dimethyl-1,4-pentadiyne E. None of the choices 3. What is the IUPAC name of the compound? (Refer to the image attached.) A. 3-Ethyl-3-hexene B. 4-Methyl-3-ethyl-3-hexene C. 3-Methyl-3-ethyl-3-hexene D. Triethylethene E. None of the choicearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





