
(a)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Grignard reagents are
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
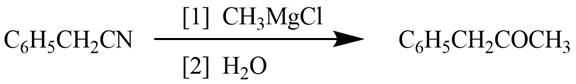
Explanation of Solution
Grignard reagents converts nitriles to corresponding carbonyl groups.
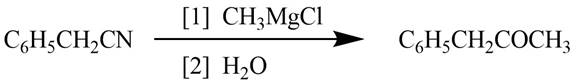
Figure 1
The reagents required to convert phenylacetonitrile
(b)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Grignard reagents are organometallic compounds having the general formula
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
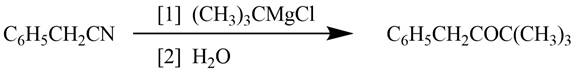
Explanation of Solution
Grignard reagents converts nitriles to corresponding carbonyl groups.
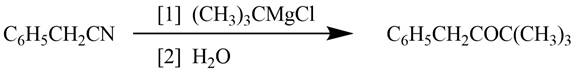
Figure 2
The reagents required to convert phenylacetonitrile
(c)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Nitrile groups get converted to carbonyl group in presence of reducing agents like DIBAL-H.
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
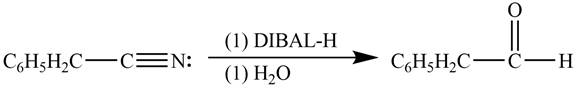
Explanation of Solution
Diisobutylaluminiumhydride(DIBAL-H) is a selective reducing agent. It readily converts nitriles to carbonyl group. DIBAL-H can be used to convert phenylacetonitrile
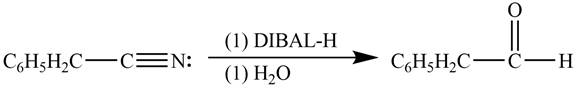
Figure 3
The reagents required to convert phenylacetonitrile
(d)
Interpretation: The reagents required to convert phenylacetonitrile
Concept introduction: Hydrolysis of nitriles in acidic medium convert them to the corresponding
Answer to Problem 22.35P
The reagents required to convert phenylacetonitrile
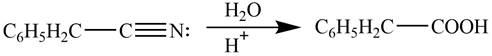
Explanation of Solution
Acidic hydrolysis of phenylacetonitrile
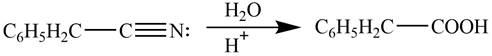
Figure 4
The reagents required to convert phenylacetonitrile
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





