
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18, Problem 12P
Interpretation Introduction
Interpretation:
To draw short sections of two parallel chains of nylon-66(each running in the same direction) and if it is possible to align them in such a way that there is hydrogen bonding between the N-H groups of one chain and the C=O groups of the parallel chain should be explained.
Concept Introduction:
A hydrogen bond is formed by a partial electrostatic attraction between hydrogen (H) atom attached to an electronegative atom (nitrogen (N), oxygen (O), or fluorine (F)) and other electronegative atom and lone pair present in the vicinity of it.
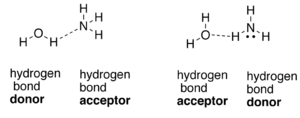
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hydrogen bonding between polyamide chains plays animportant role in determining the properties of a nylonsuch as nylon 6,6 (Table 12.6). Draw the structuralformulas for two adjacent chains of nylon 6,6 and showwhere hydrogen-bonding interactions could occur betweenthem.
Explain why aqueous NaOH solution can be stored indefinitely in polyethylene bottles, but spilling aqueous base on a polyester shirt or nylon stockings quickly makes a hole.
HHR
Polystyrene
(#6 plastic)
CH=CH;
Chapter 18 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 18.1 - Prob. 18.1QCCh. 18.4 - Problem 19-2 Complete the equation for each...Ch. 18.4 - Prob. 18.3QCCh. 18 - Prob. 1PCh. 18 - Write the IUPAC name for each compound.Ch. 18 - Prob. 3PCh. 18 - Prob. 4PCh. 18 - Prob. 5PCh. 18 - Prob. 6PCh. 18 - 0 Complete the equations for these reactions.
Ch. 18 - Prob. 8PCh. 18 - Prob. 9PCh. 18 - Prob. 10PCh. 18 - Prob. 11PCh. 18 - Prob. 12PCh. 18 - 6 Why are Dacron and Mylar referred to as...Ch. 18 - 7 What type of structural feature do the...Ch. 18 - Prob. 15PCh. 18 - Prob. 16PCh. 18 - 0 Show how triphosphoric acid can form from three...Ch. 18 - 1 Write an equation for the hydrolysis of...Ch. 18 - Prob. 19PCh. 18 - (The Pyrethrins-Natural Insecticides of Plant...Ch. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - Prob. 24PCh. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Prob. 32PCh. 18 - Prob. 33PCh. 18 - Prob. 34PCh. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - Prob. 40PCh. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Prob. 44PCh. 18 - Prob. 45PCh. 18 - Prob. 46P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nylons are strong sythetic fibers commonly used to make clothing and other goods. One of the most common nylons, Nylon 66, is made by the condensation reaction between adipic acid and hexamethylenediamine (hexane-1,6-diamine). Modify the monomers to create one repeat unit of the polymer. NH Incorrectarrow_forwardDescribe how D30 is made out of a thermosetting polymer?arrow_forwardDoes nylon6,6 contain a primary, secondary or tertiary amid group?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY