
Concept explainers
What
a. 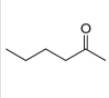 b
b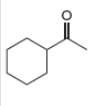 . c.
. c. 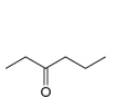 d.
d. 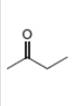
(a)
Interpretation: The alkynes that forms the given ketone as the only product after hydration with
Concept introduction: A terminal alkyne reacts with
Answer to Problem 11.36P
The alkynes that forms the given ketone as the only product after hydration with
Explanation of Solution
The alkynes that forms the given ketone as the only product after hydration with
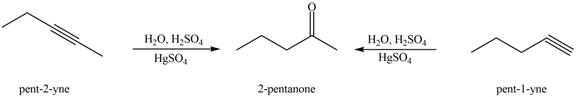
Figure 1
The terminal alkynes,
The alkynes that forms the given ketone as the only product after hydration with
(b)
Interpretation: The alkynes that forms the given ketone as the only product after hydration with
Concept introduction: A terminal alkyne reacts with
Answer to Problem 11.36P
The alkyne that forms the given ketone as the only product after hydration with
Explanation of Solution
The alkyne that forms the given ketone as the only product after hydration with
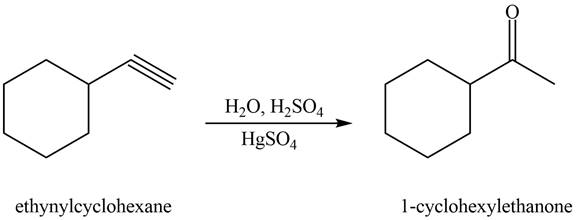
Figure 2
The terminal alkyne, ethynylcyclohexane reacts with the reagents
The alkyne that forms the given ketone as the only product after hydration with
(c)
Interpretation: The alkynes that forms the given ketone as the only product after hydration with
Concept introduction: A terminal alkyne reacts with
Answer to Problem 11.36P
The alkynes that forms the given ketone as the only product after hydration with
Explanation of Solution
The alkynes that forms the given ketone as the only product after hydration with
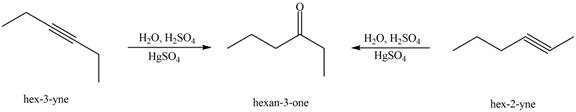
Figure 3
The terminal alkynes,
The alkynes that forms the given ketone as the only product after hydration with
(d)
Interpretation: The alkynes that forms the given ketone as the only product after hydration with
Concept introduction: A terminal alkyne reacts with
Answer to Problem 11.36P
The alkynes that forms the given ketone as the only product after hydration with
Explanation of Solution
The alkynes that forms the given ketone as the only product after hydration with
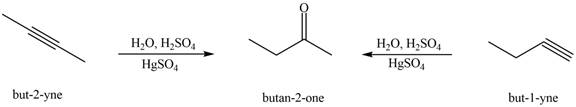
Figure 4
The terminal alkynes,
The alkynes that forms the given ketone as the only product after hydration with
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry
Chemistry & Chemical Reactivity
Chemistry: The Central Science (13th Edition)
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
- Synthesize each compound from toluene and any other organic or inorganic reagents. a. C6HSCH2BR b. C6HSCH2OC(CH3)3 -CHO с. O,N НООС -NO2 d. H,N.arrow_forwardProvide the major organic product of the following reaction. H. CN A H. CNarrow_forwardThese reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4 choices:A,DB,CA,B,CA,B,C,Darrow_forward
- 7. How would you complete the following syntheses? Provide the reagents and products of each step. OH OH ⇒e" a. b. d. H Et OMearrow_forwardWhat is the major organic product obtained from the following reaction? A. B. Yo OH OH 1. LiAlH4 2. H3O+ C. D. H OHarrow_forwardWhat is the major organic product obtained from the following reaction? A. B. HN 1. H₂NCH3, H* cat. 2. NaBH3CN C. D. HNarrow_forward
- What is the major organic product obtained from the following reaction? A. B. EN NH₂ H NH H 1. LIAIH4 2. H3O+ C. D. OH NH₂ ENarrow_forwardWhat is the major organic product obtained from the following reaction? A. CI B. H 1. (CH3CH₂)2CuLi 2. H3O+ C. D. •ay.a OHarrow_forward6. Provide the major organic product for the following reaction. HN Br COOH H3CO 1. LIAIH4 2. HCI, H₂Oarrow_forward
- What is the major organic product obtained from the following reaction? NOH HO 1 mol H20 cat. H;SO CEN `NH2 NH2 3 а. 1 b. 2 C. 3 d. 4 2.arrow_forwardWhat is the major organic product obtained from the following reaction? A. B. NH₂ OH 1. LIAIH4 2. H3O+ NH₂ C. D. NH NH₂arrow_forwardWhat is the major organic product obtained from the following reaction? A. B. HN il ΗΝ 'N' N C. D. Narrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





