
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 3E
Consider the incomplete valence shell representation below.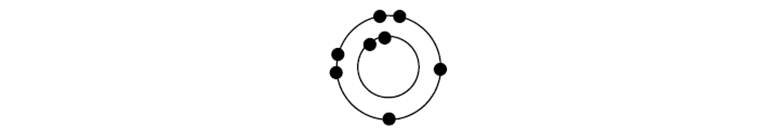
a. Assume the atom is neutral, and write the correct nuclear charge at the center of the atom.
b. What is the identity of this neutral atom?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Completely answer the following. Write legibly or typewrite the answers.
1. Discuss the evolution of the “models” of an Atom. Be concise.
A polar covalent bond between two atoms occurs whena. one atom attracts shared electrons more strongly than another atom.b. atoms attract electrons equally.c. an electron from one atom is completely transferred to another atom.d. the molecule becomes ionized.e. a hydrogen atom is shared between two different atoms
In the nitric oxide molecule (NO), which atom is expected to hold the partial negative charge?
A. the N atom
B. the O atom
C. neither atom holds a partial negative charge
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. What is ionization energy? How does it change as you go across a row in the periodic table? Be sure to use the coulombic attraction between electrons and the nucleus in your explanation. 2. How does ionization energy change as you go down a group in the periodic table? Be sure to use the coulombic attraction between electrons and the nucleus in your explanation.arrow_forwardHow many electrons Magnesium atom must, lose/gain to become stable? What charge would it obtain?arrow_forwardNegatively charged atoms are called cations. A. True B. Falsearrow_forward
- What subatomic particles diated whether a bond is covalent or ionic? A. Electrón b. Alpha particle c. Photon d. Lightarrow_forwardYour answers are saved automatically. Remaining Time: 52 minuțes, 59 seconds. * Question Completion Status: A Moving to another question will save this response. Question 20 Determine the number of electrons that the four elements below gain or lose to obtain the nearest noble gas configurations of electrons. hydrogen, Lithium, nitrogen, oxygen Hydrogen A. gain 1 electron v Lithium B. lose 2 electrons v Nitrogen C. gain 2 electrons v Oxygen D. lose three electrons E. lose 1 electron F. gain 3 electrons Moving to another question will save this response. 23 257 I 44,823 APRarrow_forwardTo complete a dot diagram for an ion. Cations... A would gain enough electrons to have a full eight. B. Would not change its number of electrons C. Would lose all of its valence electronsarrow_forward
- Which of these elements would be predicted to form an anion when ionized? a.) sodium b.) barium c.) oxygen d.) aluminumarrow_forward1.Explain the following Why cations are positively charged and Anions are negatively charged 2.who is againing and who is lossing electronsarrow_forward1. The higher the number of electrons in an atom, the higher the effective nuclear charge will be. - True - False 2.The Bohr atomic model states that electrons move from one orbit to another orbit when they absorb or emit photons - True - False 3. Which of the following compounds is a possible combination of a metal and nonmetal taking into account their stable ions? a.NaI2 b.CaF3 c.MgBr d.Al2S3 4. Which of the following compounds has contains the strongest bond? a.CH4 b.C2H2 c.C2H4 d.CH3Clarrow_forward
- 18. The inner electrons of an atom that do not participate in chemical bonding are referred to as what kind of electron A. valence electrons B. ground state electrons C. reactant D. core electrons E. octet electronsarrow_forwardWhich ones are singly charged anions? (Choose all the correct answers) Select one or more: a. chlorite b. chromate c. dihydrogen phosphate d. hydrogen phosphate e. cyanide f. hydrogen sulfate g. acetate h. sulfite i. sulfate j. peroxidearrow_forwardCHEMISTRY 101 LECTURE NOTES 4. Give one example (atomic symbol and name) for each of the following. a. a main-group (representative) element in the second period b. an alkali metal c. a transition element in the fourth-period d. a lanthanide element 5. Write the formula for the compound of each of the following pairs of ions. a. Fe and CN b. K' and SO,² c. Li' and N³ d. Ca²² and P e. NH, and PO, 6. The following table gives numbers of electrons, protons, and neutrons in atoms or ions of a number of elements. Answer the following: (a) Which of the species are neutral? (b) Which are negatively charged? (c) Which are positively charged? (d) What are the conventional symbols for all the species? Atom or lon of Elenment B Number of electrons 10 18 28 Number of protons Number of neutrons 36 5. フ 19 30 35 20 36 46 7. Consider a hypothetical case in which the charge on a proton is twice that of an electron. Using this hypothetical case, and the fact that atoms maintain a charge of 0, how many…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY