
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 14.28P
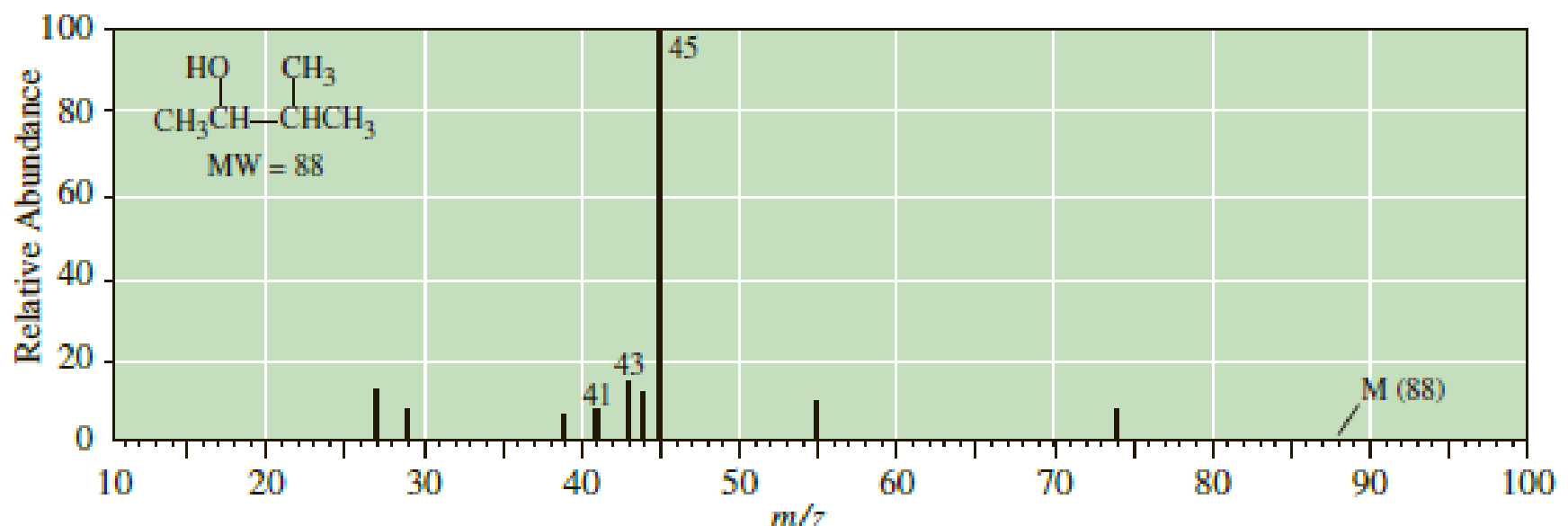
Following is the mass spectrum of 3-methyl-2-butanol. The molecular ion m/z 88 does not appear in this spectrum. Propose structural formulas for the cations of m/z 45, 43, and 41.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The mass spectrum of an aldehyde shows a parent peak at m/z = 58 and a base peak at m/z = 29. Propose a structure, and identify the
two species whose m/z values were listed.
Name the compound in the box below.
A compound containing only carbon, nitrogen, oxygen, and hydrogen contains four carbon atoms. If the M+ peak in its mass spectrum appears at m/z = 87, then how many nitrogen atoms does it contain?
c) The mass spectrum of 2,3-dibromopentane (CH; CHBr CHBr CH₂ CH3) includes the following peaks.
Mass number (m/z) Relative abundance
15
29
107
109
199
201
203
i. What is the mass number of molecular ion, [CH; CHBr CHBr CH₂ CH₂]? Show your working.
ii. Identify the molecular formula (including isotopic composition where relevant) of the 6 peaks.
a
b
d
22
e
f
Mass number (m/z)
15
29
107
109
199
100
39
44
45
0.3
0.6
0.3
201
203
Molecule formula
[CH₂]
Chapter 14 Solutions
Organic Chemistry
Ch. 14.2 - Calculate the nominal mass of each ion. Unless...Ch. 14.3 - Propose a structural formula for the cation at m/z...Ch. 14.3 - The low-resolution mass spectrum of 2-pentanol...Ch. 14 - Draw acceptable Lewis structures for the molecular...Ch. 14 - The molecular ion for compounds containing only C,...Ch. 14 - For which compounds containing a heteroatom (an...Ch. 14 - The so-called nitrogen rule states that if a...Ch. 14 - Prob. 14.8PCh. 14 - Prob. 14.9PCh. 14 - Prob. 14.10P
Ch. 14 - Determine the probability of the following in a...Ch. 14 - The molecular ions of both C5H10S and C6H14O...Ch. 14 - Prob. 14.13PCh. 14 - Carboxylic acids often give a strong fragment ion...Ch. 14 - For primary amines with no branching on the carbon...Ch. 14 - Prob. 14.16PCh. 14 - A characteristic peak in the mass spectrum of most...Ch. 14 - Predict the relative intensities of the M and M +...Ch. 14 - The mass spectrum of compound A shows the...Ch. 14 - The mass spectrum of compound B, a colorless...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - Write molecular formulas for the five possible...Ch. 14 - The molecular ion in the mass spectrum of...Ch. 14 - Prob. 14.24PCh. 14 - Following is the mass spectrum of 1-bromobutane....Ch. 14 - Following is the mass spectrum of...Ch. 14 - Following is the mass spectrum of an unknown...Ch. 14 - Following is the mass spectrum of...Ch. 14 - Prob. 14.29PCh. 14 - Following are mass spectra for the constitutional...Ch. 14 - 2-Methylpentanal and 4-methyl-2-pentanone are...Ch. 14 - Prob. 14.32PCh. 14 - Account for the presence of the following peaks in...Ch. 14 - All methyl esters of long-chain aliphatic acids...Ch. 14 - Propylbenzene, C6H5CH2CH2CH3, and isopropyl...Ch. 14 - Account for the formation of the base peaks in...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
16.43 The following pictures represent solutions at various stages in thetitration of a weak diprotic acid with...
Chemistry (7th Edition)
Problem 11.1 Neopheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a)...
Organic Chemistry
Give the IUPAC name for each compound.
Organic Chemistry
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is the mass spectrum of bromocyclopentane. The molecular ion m/z 148 is of such low intensity that it does not appear in this spectrum. Assign structural formulas for the cations of m/z 69 and 41.arrow_forwardFollowing is the mass spectrum of an unknown compound. The two highest peaks are at m/z 120 and 122. Suggest a structure for this compound. (Data from http://webbook.nist.gov/chemistry/.)arrow_forwardWrite molecular formulas for compounds that show the following molecular ions in their high-resolution mass spectra, assuming that C, H, N, and O might be present. The exact atomic masses are: 1.007 83 (1H), 12.000 00 (12C), 14.003 07 (14N), 15.994 91 (16O). (a) M+=98.0844 (b) M+=123.0320arrow_forward
- The mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. One appears at m/z = 111 and the other appears at m/z = 97. Determine the identity and structure of each of these fragmentsarrow_forwardThe mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. Kne appears af m/z=111 and the other appears at m/z=97. Identify the structure of each of these fragments.arrow_forwardAssigning Possible Structures to Fragments in a Mass Spectrum The mass spectrum of 2,3-dimethylpentane [(CH3)2CHCH(CH3)CH2CH3] shows fragments at m/z = 85 and 71. Propose possible structures for the ions that give rise to these peaks.arrow_forward
- A mass peak at m/z = 59 appears in the mass spectrum of an amide, C5H11NO. Draw the structure of a molecule that is consistent with this result.arrow_forwardFollowing is the mass spectrum of 3-methyl-2-butanol. The molecular ion mlz 88 does not appear in this spectrum. Propose structural formulas for the cations of mlz 45, 43, and 41. 100 45 CH3 CH,CH-CHCH, MW - 88 но 80 60 20 M (88) 10 20 30 40 50 60 70 80 90 100 Relative Abundancearrow_forwardA mass spectrum shows significant peaks at m>z = 87, 115, 140, and 143. Which of the following compounds is responsible for that mass spectrum?arrow_forward
- Following is the mass spectrum of an unknown compound. The two highest peaks are at mlz 120 and 122. Suggest a structure for this compound. (Data from http://webbook .nist.gov/chemistry/.) 100 41 80 20 120 122 0 rt 10 20 30 40 60 70 110 140 80 90 m/z 50 100 120 130 150 160 Relative Abundancearrow_forwardLabel the molecular ion, the base peak, and the M + 1 peak in the mass spectrum of pentane (C5H12).arrow_forwardCO9TO9Q4167 The mass spectrum of an unknown compound has a molecular ion peak with a relative intensity of 54.77% and an M+1 peak of 6.66%. How many carbon atoms are in the compound? (Fill in an integer number)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY