
Figure 5.12 A doctor injects a patient with what the doctor thinks is an isotonic saline solution. The patient dies, and an autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?
To analyze:
The type of the solution inserted in the body of the patient, which causes the destruction of the RBCs.
Introduction:
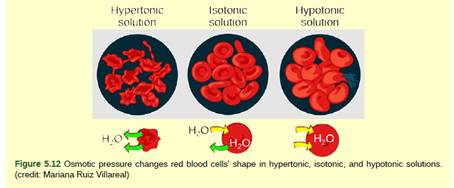
Solutions are of three types depending upon the concentration of solutes. The solution having a high concentration of solutes is called hypertonic solution, the one with less solute is called hypotonic solution and two solutions having equal concentration of solutes is known as an isotonic solution.
Explanation of Solution
The solution which is injected into a patient was not isotonic but hypotonic. A hypotonic solution is the one which has less solute and more solvent (water) concentration. Due to this, the solvent rushes inside the RBC by the process called as diffusion. When the water rushes in the RBCs, the cells swell and burst.
Diffusion of solutes and water takes place from high concentration to low concentration. The RBCs, when placed in the hypotonic solution swell and burst, which causes the destruction of RBCs.
Want to see more full solutions like this?
Chapter 5 Solutions
Biology 2e
Additional Science Textbook Solutions
College Physics
Campbell Biology in Focus (2nd Edition)
Human Anatomy & Physiology
Campbell Essential Biology with Physiology (5th Edition)
Concepts of Genetics (12th Edition)
Campbell Essential Biology (6th Edition) - standalone book
- Figure 3.22 A doctor injects a patient with what he thinks is isotonic saline solution. The patient dies, and autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?arrow_forwardWhy is it advantageous for the cell membrane to be fluid in nature?arrow_forwardWhich answer is correct (a or b) for the question about why your hands and feet get wrinkled after staying in the bath for too long. a) the water and dissolved particles are drawn out of your skin by diffusion into the large body of water around you. b) the water is actually drawn into your skin by osmosis. explain why (a or b) is correct.arrow_forward
- Isotonic saline and 5% dextrose in water are solutions considered isotonic to human blood. What effect on red blood cells would you expect if a patient were given these fluids intravenously? A solution of 10% dextrose in water is hypertonic to human blood.arrow_forwardA patient has had a serious accident and lost a lot of blood. In an attempt to replenish body fluids, a large amount of distilled water was transferred directly into one of his veins. What will be the most probable result of this transfusion? It will have serious, perhaps fatal, consequences because the red blood cells will be hypertonic relative to the body fluids and the cells will burst. It will have serious, perhaps fatal, consequences because the red blood cells will be hypotonic relative to the body fluids and the cells will shrivel. It will have no unfavorable effect as long as the water is free of viruses and bacteria. It will have no serious effect because the kidneys would quickly eliminate the excess water. It will have serious, perhaps fatal, consequences because there will be too much fluid for the heart to pump.arrow_forwardIn medicine, why is it important to administer only isotonic intravenous solutions to patients? Example of isotonic IV fluids are normal saline and lactated Ringer’s solution.arrow_forward
- Inflammatory responses are associated with lots of swelling, where fluids rush into cells and surrounding tissues. To help quickly relieve swelling, what type of IV solution could a doctor administer to her patient? hypotonic hypertonic isotonicarrow_forwardIsotonic, hypotonic, and hypertonic IV (intravenous) fluid solutions are widely used in the healthcare setting. As a possible health care professional, you must know how each of the solutions work on the body and why they are given. a) When does each type of solution must be applied to a patient? b) What does each type of solution do the cells? Isotonic IV fluid solution Hypotonic IV fluid solution Hypertonic IV fluid solution|arrow_forwardYour friend notes that Gatorade has electrolytes like sodium and potassium chloride. He figures that if a few electrolytes are good for you, then a lot must be even better. Following this logic, he adds nearly a tablespoonful of salt to his Gatorade. What kind of solution is his Gatorade now (hypotonic, isotonic, or hypertonic)? What will likely happen to his cells if he drinks this liquid?arrow_forward
- Why is it important to know the isotonic point of human cells when administering an IV? Explain what would happen to the red blood cells if an IV solution were hypotonic or hypertonic.arrow_forwardYou are a doctor in the emergency room. You have a patient whose blood cells are composed of the following: 80 molecules of glucose, 50 molecules of hemoglobin, 20 molecules of dextrose, and 2000 molecules of water. a. What is the total solute concentration of her cells? b. If you needed to make an isotonic solution of saline for her IV, how many salt molecules would you need to add to 1000 molecules of water? How do you know this is the correctamount?arrow_forwardMany old time folk remedies made use of the priciples of diffusion and osmosis. Suppose you had a sliver of wood or another small foreign object embedded under your skin. It is slightly swollen and hurts. Your grandma or older neighbor tells you to soak the affected part in salt water. Using your knowledge of diffusion/osmosis, how do you think this would affect the injury? Will it help you get the sliver out?arrow_forward
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

