
Concept explainers
Fill in the names beside the symbols of the following elements commonly found in living matter.
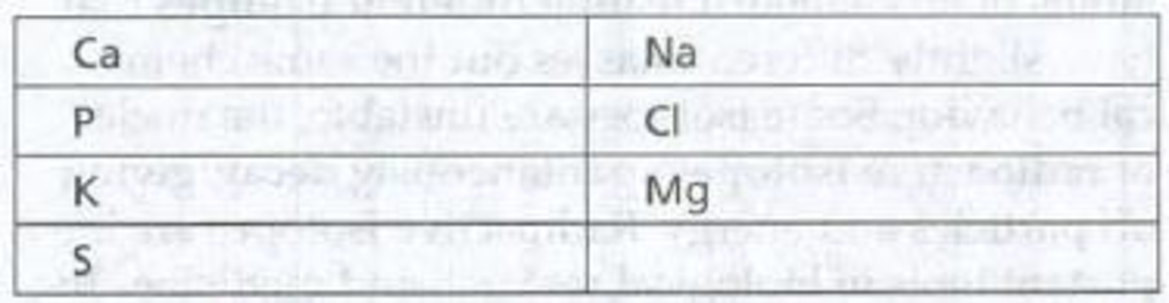
To fill: The names beside the symbol of the given element that are commonly found in living matter.
Introduction: In the simplest level of organization, the human body consists of various chemical structures. The substances that cannot be broken down into simpler substances by ordinary chemical reactions are called elements. Every element has its own chemical symbol.
Answer to Problem 1IQ
Tabular representation: The names of the symbol of the given element are in Table 1.
Table 1: Names of the symbol of the given element
| Ca -Calcium | Na- Sodium |
| P- Phosphorus | Cl- Chlorine |
| K- Potassium | Mg- Magnesium |
| S- Sulfur |
Explanation of Solution
Chemical elements are represented as symbols. In general, first one or two letters of element’s name are represented as a symbol of an element. For example, sulfur is represented as symbol “S” and calcium is represented as “Ca”. But few elements have symbols that have no relationship with their names. For example, potassium is represented by symbol “K” and sodium is represented as “Na”. For such cases, origin of the elements is used.
- Potassium- K- Latin name Kalium –means alkali
- Sodium- Na- Latin name for sodium carbonate- Natrium
Want to see more full solutions like this?
Chapter 2 Solutions
Study Guide for Campbell Biology
- Identify all of the chirality centers in the structure. The chirality centers are: A В C D `NH E F OH b H Он I J K L M N P Q Rarrow_forwardHow many grams of glucose (C6H2O6 molecular mass =180daltons) would be present in one liter of a 1M (molar) solution of glucose?arrow_forwardThe fundamental unit of matter, both in living and non-living matter, is thearrow_forward
- IDENTIFY THE FOLLOWING PARTS - A B C D E F G H I J Karrow_forwardBRIEFLY DESCRIBE each part of the structure (see attached photo)arrow_forward(a) Identify a phospholipid from the list of compounds shown above. __________ (b) Identify a triglyceride from the list of compounds shown above. ___________(c) Identify a glycolipid from the list of compounds shown. _________ Write NA if structure is not found.arrow_forward
- Consider these compounds: A. PbBr, B. MnS C. Ag,CO3 D. AIPO, Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the description, include all the relevant compounds by writing your answer as a string of characters without punctuation, e.g, ABC.) Without doing any calculations it is possible to determine that magnesium fluoride is more soluble than and magnesium fluoride is less soluble than| It is not possible to determine whether magnesium fluoride is more or less soluble than by simply comparing Kgp values.arrow_forwardнннн ннннН с-С-с-с-с-С-С-С-С-с-н HHHH HHHH H H. Ising the graphic above, answer the following question. orrectly identify the above molecule. s this molecule solid or liquid at room temperature? Name a major source of this macromolecule.arrow_forwardDetermine the chemical formula for each of the following carbohydrates. Use the ratio for carbohydrates to fill in the missing number in the chemical formula. Example: C3H?O3 = C3H6O3 1:2:1 ratio Carbon 1 x 3 = 3 Hydrogen 2 x 3 = 6 Oxygen 1 x 3 = 3 C6H__O6 C5H__O5 C7H__O7 C4H__O4arrow_forward
- Solid iron(II) fluoride (FeF2, Ksp= 2.36 x 10-6) is dissolved in water. If 8.1 x 10-3 mol L-1 of iron(II) ion is found to be in solution. Is the solution saturated, unsaturated, desaturated or supersaturated.arrow_forwardElemental analysis of a compound with molar mass 342.3 g/mol gives the following mass percent composition: C 42.11%, H 6.48%, O 51.41%. Find the molecular formula of the compound. Enter your answer in the space below using the following format: if the molecular formula of a compound containing elements X, Y, and Z is X2YZ3 enter your answer as X2YZ3.arrow_forwardCompound P was discovered by a scientist. Compound P is a dipeptide, optically active and has the molecular formula C„H14N2O3. Compound P is formed when compound Q and compound R joined together by condensation reaction. While, monomers S and T are formed by modifying the compounds Q and R. Polymer U is formed by the condensation reaction of monomers S and T. Draw the possible structural formulae of compounds P, Q, R, S, T and U. Label the peptide bond(s) for compound P. Draw the possible structural formulae for repeating unit of polymer U. Please state the number of functional groups present in compound P.arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

