Organic chemistry Iodoethane does not react via Sn2 reaction with NaI in Acetone. It is a good leaving group and on the primary carbon, why doesn't it react Sn2? (I know it reacts Sn1)
Organic chemistry Iodoethane does not react via Sn2 reaction with NaI in Acetone. It is a good leaving group and on the primary carbon, why doesn't it react Sn2? (I know it reacts Sn1)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.49P
Related questions
Question
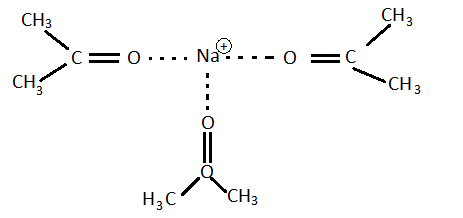
Iodoethane does not react via Sn2 reaction with NaI in Acetone. It is a good leaving group and on the primary carbon, why doesn't it react Sn2? (I know it reacts Sn1)
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning