Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter22: Biochemistry
Section: Chapter Questions
Problem 63E
Related questions
Question
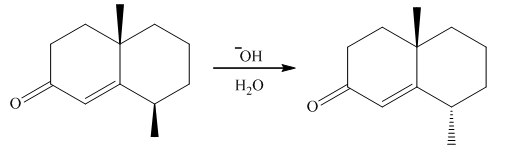
Treatment of α,β-unsaturated carbonyl compound X with base forms the diastereomer Y. Write a stepwise mechanism for this reaction. Explain why one stereogenic center changes configuration but the other does not.

Transcribed Image Text:"OH
H20
Y.
Expert Solution
Step 1
The provided reaction is shown as follows:
Step 2
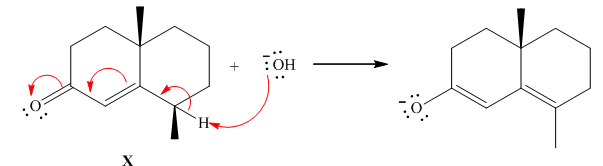
The Stepwise mechanism for the provided reaction is shown as follows:
The base abstracts the proton from gamma-hydrogen of α,β- unsaturated compound X gives an enolate anion.Thus ,it is shown as follows:
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning