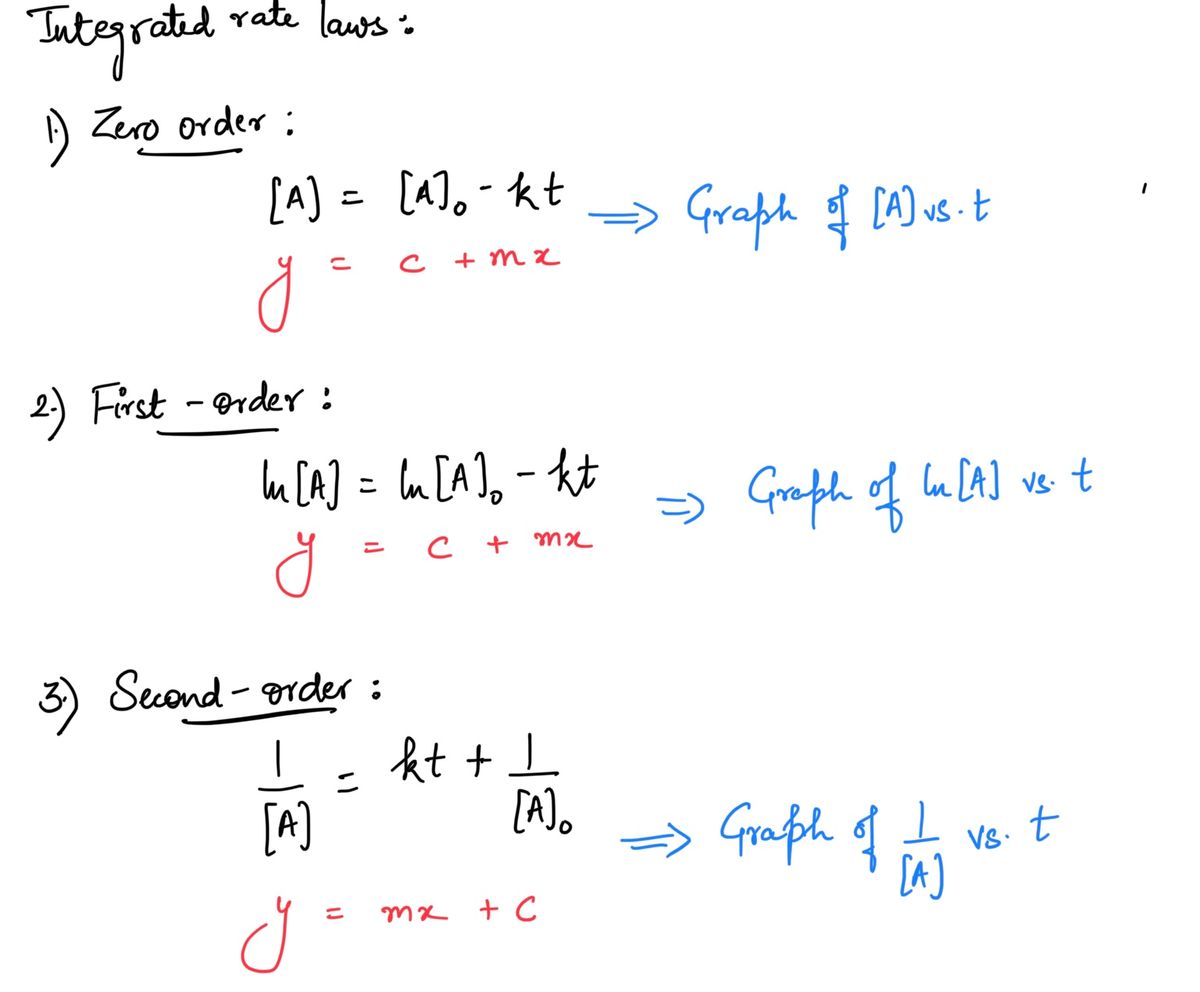
Moving to another question will save this Question 20 A graph that displays a straight line when the reciprocal of the concentration is plotted as a function of time. The graph has a slope of 0.482 and a y- intercept of 0.756. What is the rate constant? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation-examples include 1.23 and 12.3 and 120. and -123)
Moving to another question will save this Question 20 A graph that displays a straight line when the reciprocal of the concentration is plotted as a function of time. The graph has a slope of 0.482 and a y- intercept of 0.756. What is the rate constant? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation-examples include 1.23 and 12.3 and 120. and -123)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section14.1: Rates Of Chemical Reactions
Problem 14.1CYU: Sucrose decomposes to fructose and glucose in acid solution. A plot of the concentration of sucrose...
Related questions
Question

Transcribed Image Text:AMoving to another question will save this res
Question 20
A graph that displays a straight line when the reciprocal of the concentration is plotted as a function of time. The graph has a slope of 0.482 and a y-
intercept of 0.756. What is the rate constant?
(the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation-examples include 1.23 and 12.3 and 120. and
-123)
Question 20 of 29
AMoving to another question will save this response
PIL
esc
%23
%24
4
&
7.
8
delete
R
T.
Y
TO
tab
enter
D.
F
K
return
caps lock
C
V
elt
control
option
command
option
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning