Question 2 In class, we defined as the effective resolution for counting positions of non-interacting particles moving in three dimensions. Why is this the minimum resolution that we consider? O λ is defined as the size of the particle. We cannot have microstates smaller than the size of the particle. O A is a made up resolution that helps with the math. O For an ideal gas, if particles get too close together, we need to start considering particle-particle interactions. 1 pts O The Heisenberg uncertainty principle dictates our minimum resolution because it is difficult to precisely know the velocity and position of particles below a certain limit.
Question 2 In class, we defined as the effective resolution for counting positions of non-interacting particles moving in three dimensions. Why is this the minimum resolution that we consider? O λ is defined as the size of the particle. We cannot have microstates smaller than the size of the particle. O A is a made up resolution that helps with the math. O For an ideal gas, if particles get too close together, we need to start considering particle-particle interactions. 1 pts O The Heisenberg uncertainty principle dictates our minimum resolution because it is difficult to precisely know the velocity and position of particles below a certain limit.
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
It looks like you may have submitted a graded question that, per our Honor Code, experts cannot answer. We've credited a question to your account.
Your Question:
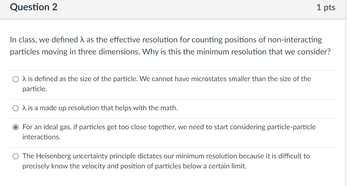
Transcribed Image Text:Question 2
In class, we defined as the effective resolution for counting positions of non-interacting
particles moving in three dimensions. Why is this the minimum resolution that we consider?
O λ is defined as the size of the particle. We cannot have microstates smaller than the size of the
particle.
O A is a made up resolution that helps with the math.
O For an ideal gas, if particles get too close together, we need to start considering particle-particle
interactions.
1 pts
O The Heisenberg uncertainty principle dictates our minimum resolution because it is difficult to
precisely know the velocity and position of particles below a certain limit.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
