HE154 S20 Ch17 Sec7-9 Later iCloud Preferences... r Practice 17.12- Enhanced - with Feedback • Part A Find the [OH-] of a 0.50 M pyridine (C5 H5N) solution. (The value of K for pyridine (C5 H,N) is 1.7 x 10-9.) Express your answer to two significant figures and include the appropriate units. НА [ОН ]- Value Units %3D Submit Request Answer v Part B Find the pH of a 0.50 M pyridine (C5 H5N) solution. HẢ [OH-] = Value Units %3D Submit Request Answer Part B Find the pH of a 0.50 M pyridine (C5 H; N) solution. Express your answer using two decimal places. nν ΑΣφ pH = %3D Submit Request Answer Provide Feedback
HE154 S20 Ch17 Sec7-9 Later iCloud Preferences... r Practice 17.12- Enhanced - with Feedback • Part A Find the [OH-] of a 0.50 M pyridine (C5 H5N) solution. (The value of K for pyridine (C5 H,N) is 1.7 x 10-9.) Express your answer to two significant figures and include the appropriate units. НА [ОН ]- Value Units %3D Submit Request Answer v Part B Find the pH of a 0.50 M pyridine (C5 H5N) solution. HẢ [OH-] = Value Units %3D Submit Request Answer Part B Find the pH of a 0.50 M pyridine (C5 H; N) solution. Express your answer using two decimal places. nν ΑΣφ pH = %3D Submit Request Answer Provide Feedback
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 13E: For a titration to be effective, the reaction must be rapid and the yield of the reaction must...
Related questions
Question
![HE154 S20 Ch17 Sec7-9
Later
iCloud Preferences...
r Practice 17.12- Enhanced - with Feedback
• Part A
Find the [OH-] of a 0.50 M pyridine (C5 H5N) solution. (The value of K for pyridine (C5 H,N) is 1.7 x 10-9.)
Express your answer to two significant figures and include the appropriate units.
НА
[ОН ]-
Value
Units
%3D
Submit
Request Answer
v Part B
Find the pH of a 0.50 M pyridine (C5 H5N) solution.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8ddcfb62-a75f-441b-9ba0-61b722369038%2Fe28e9e16-5f2d-45a0-a634-996a21df13d2%2F27qwgam.jpeg&w=3840&q=75)
Transcribed Image Text:HE154 S20 Ch17 Sec7-9
Later
iCloud Preferences...
r Practice 17.12- Enhanced - with Feedback
• Part A
Find the [OH-] of a 0.50 M pyridine (C5 H5N) solution. (The value of K for pyridine (C5 H,N) is 1.7 x 10-9.)
Express your answer to two significant figures and include the appropriate units.
НА
[ОН ]-
Value
Units
%3D
Submit
Request Answer
v Part B
Find the pH of a 0.50 M pyridine (C5 H5N) solution.
![HẢ
[OH-] =
Value
Units
%3D
Submit
Request Answer
Part B
Find the pH of a 0.50 M pyridine (C5 H; N) solution.
Express your answer using two decimal places.
nν ΑΣφ
pH =
%3D
Submit
Request Answer
Provide Feedback](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8ddcfb62-a75f-441b-9ba0-61b722369038%2Fe28e9e16-5f2d-45a0-a634-996a21df13d2%2Farfrn7.jpeg&w=3840&q=75)
Transcribed Image Text:HẢ
[OH-] =
Value
Units
%3D
Submit
Request Answer
Part B
Find the pH of a 0.50 M pyridine (C5 H; N) solution.
Express your answer using two decimal places.
nν ΑΣφ
pH =
%3D
Submit
Request Answer
Provide Feedback
Expert Solution
Given Information:
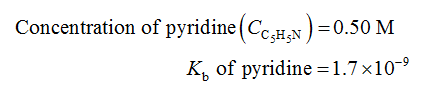
Calculations:
Part A
Pyridine is a weak base. Therefore, according to acid-base theory, the concentration of hydroxide ion [-OH] is calculated by the formula shown below.
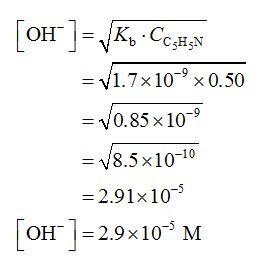
Part B
The pOH is calculated as shown below.
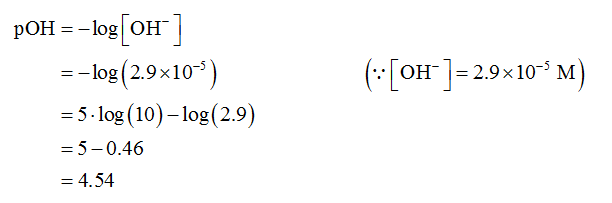
The pH is calculated as shown below.
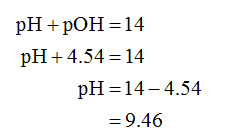
The pH of 0.50 M pyridine is 9.46.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax
