Formula Lewis structure VSEPR model Geometry name AB(E) notation 1.C2H6 H H-C ·C-H H H 2. C2H4 3. C2H2 +2F10 4. CH3CH2OH 5. CHCl3 6. HCN 7. H2CO I H Polarity (P or NP) NP NP NP 8. H₂O2 2+12 14 9. H₂S H 〇: 14 H 14 P 10. CO₂
Formula Lewis structure VSEPR model Geometry name AB(E) notation 1.C2H6 H H-C ·C-H H H 2. C2H4 3. C2H2 +2F10 4. CH3CH2OH 5. CHCl3 6. HCN 7. H2CO I H Polarity (P or NP) NP NP NP 8. H₂O2 2+12 14 9. H₂S H 〇: 14 H 14 P 10. CO₂
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter9: Liquids And Solids
Section: Chapter Questions
Problem 47QAP
Related questions
Question
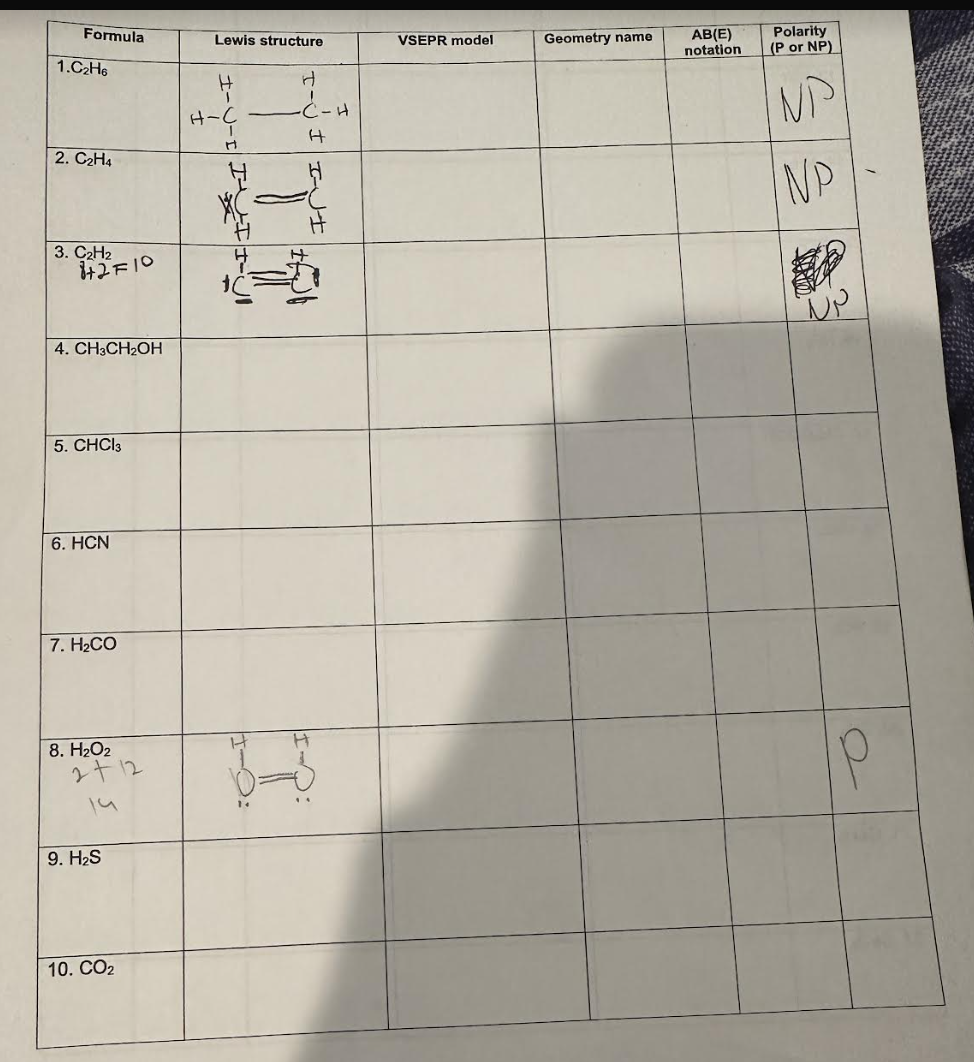
Transcribed Image Text:Formula
Lewis structure
VSEPR model
Geometry name
AB(E)
notation
1.C2H6
H
H-C
·C-H
H
H
2. C2H4
3. C2H2
+2F10
4. CH3CH2OH
5. CHCl3
6. HCN
7. H2CO
I
H
Polarity
(P or NP)
NP
NP
NP
8. H₂O2
2+12
14
9. H₂S
H
〇:
14
H
14
P
10. CO₂
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning