Q: Can the molecule on the right-hand side of this organic reaction be made in good yield from no more…
A: Given is organic compound.Given compound is fused ring compound. As we can see one six member ring…
Q: Question 23 Identify the Major and ALL Minor product(s) that are expected for each of the following…
A:
Q: н. NaOH 20
A: Given,The reaction is:
Q: QUESTION 6 Identify the salts that will not hydrolyze water. The solution will be neutral. NaCl NH41…
A: Answer:Salts of strong acid and strong base doesn't get hydrolyzed in water. So, in this problem we…
Q: HCN is a weak acid (K₁ = 6.20 x 10-10), so the salt, KCN, acts as a weak base. What is the pH of a…
A:
Q: For each of the following acid-base reactions, calculate how many grams of each acid are necessary…
A: The objective of the question is to calculate the amount of acid (HNO3) in grams required to…
Q: 7. What two reagents would have been reacted together in order to obtain the following alkene via a…
A: In a wittig reaction an aldehyde or ketone reacts with phosphonium ylide to produce alkene
Q: Draw a structural formula for the intermediate in the following reaction: CH3CH2CH=CHCH2CH2CH2CH3 +…
A: Information about the question
Q: The following H-NMR spectrum (showing all available signals) is for one of the possible products…
A: A triplet at 1.1 ppm and a quartet at 2.4 ppm suggests that the molecule has -CH2CH3 group. The…
Q: 6) What is the product of the following reaction? CH A) CHOCH LOCH OCH CH₂OCH сно
A: It is an example of nucleophilic substitution reactionnucleophile can attack at 2nd or 4th position.…
Q: bond order for B2^- please draw the picture and explain what I am doing wrong. I am getting six…
A: Answer of the given question is given in below section.Explanation:Step 1:
Q: Determine concentration of hydroxide ion, OH, in a solution of HNO₂ by constructing an ICE table,…
A: Answer:Upon adding in water weak acid gets partially ionized and an equilibrium between its ionized…
Q: Use bond enthalpies in the table below to estimate AH for each of the following reactions. Average…
A: Given,The reactions:
Q: 1 2 3 or or 4
A: Two esters compound react in the presence of a strong base. The reaction produces a beta-keto ester
Q: 5.5 Stereoisomeric Relationships: Enantiomers and Diastereomers Identify the relationship between…
A: The chiral compounds that are non superimposable on its mirror images and have opposite…
Q: Part D Η Spell out the full name of the compound.
A: Find out the name of this compound
Q: What are some identifying characteristics (reactants, products, formats) for each of the 8 reaction…
A: The eight types of chemical reactions are synthesis, decomposition, single displacement, double…
Q: 4) Consider the following reactions that are either Diels-Alder or rely on a Diels-Alder step: a)…
A: In a Diels-Alder reaction a conjugated diene and a dienophile undergo [4+2]cycloaddition reaction…
Q: Consider the reaction below. Then select all that are true about this reaction. CH,CH₂ÖH H CH,CH,OH…
A: Answer:In any reaction, specie that loses its H+ ion is called Bronsted-Lowry acid and specie that…
Q: Use the standard reduction potentials located in the 'Tables' linked above to calculate the…
A:
Q: Select the correct IUPAC name for the cycloalkane:
A: The IUPAC nomenclature provides a set of rules by which an organic compound can be named…
Q: ct Question 4 0 / 9 pts Sulfuric acid reacts with sodium hydroxide according to this reaction: H₂SO4…
A: The objective of the question is to calculate the molarity of the sulfuric acid (H2SO4) solution.…
Q: 0 EtO (racemic) EtO
A: We have to choose the correct option.
Q: Identifying the major species in weak acid or weak base equilibria The preparations of two aqueous…
A: Given,0.36 mol HCl is added to 1.0 L of a 1 M NH3 solutionacids:bases:other:0.1 mol of HI is added…
Q: 15. A compound, C7H14O, gives rise to the 13C and 1H NMR data shown below. Draw its structure. 13C…
A: we calculated the degree of unsaturation.DoU = Nc+1 - N(H)/2 = (7+1)- (14)/2 =…
Q: 3 reaction that forms a cyclic ether. OH H2SO4 OH + H₂O
A:
Q: PART 1: For the decalin derivative below please identify the correct Newman projection: Please type…
A: The planar decalin structure is converted to chair structure and then easily the Newmann projection…
Q: The concentration of the stock glucosamine solution is 100 mM. A student took 0.5 ml of this stock…
A: “Since you have posted multiple questions, we will provide the solutiononly to the first question as…
Q: Identify the number of stereoisomers expected for the following:
A: A pair of substances which are non-super imposable mirror images are called enantiomers.A pair of…
Q: The green band consisted of chlorophylls. What does it tell you about the relative polarity of the…
A: Here, the elution behavior of compounds in the chromatography is influenced by the polarity of the…
Q: 2.93 g Measured Volume of Water: 100.0 mL each question with correct significant figures and units.…
A: Given,Measured mass of NaCl = 2.93 gMeasured Volume of Water = 100.0 mLmolar mass of NaCI = 58.44…
Q: Question 6. Predict the product of the following reaction and draw the mechanism. Includes all…
A: In electrophilic aromatic substitution, the hydrogen of the benzene is replaced by an electrophile.
Q: Br OH OH Movie:
A: Grignard Reaction: Firstly react bromobenzene with magnesium (Mg) metal in dry ether to form…
Q: Phthalonitrile (C2H4N2) is produced by the ammoxidation of oxylene (C 8H10) according to the…
A: The balanced equation for givenreaction is C8H10(l) + 3O2(g) + 2NH3 (g) → C8H4N2 (s) + 6H2O(l)moles…
Q: Part A Which of the following compounds would react with Tollens' reagent, Benedict's reagent, both,…
A: Answer:Different tests are used to differentiate between aldehydes and ketones. In the given problem…
Q: Which of the following mathematical expressions is the Henderson-Hasselbalch equation? 'a.¤ 'b.¤…
A: Answer Explanation:Step 1: Step 2: Step 3: Step 4:
Q: Name: Give the IUPAC name for each compound. Part 1 of 2 Give detailed Solution with explanation…
A: IUPAC nomenclature are the set of rules that are used or taken into consideration while naming any…
Q: Based on the guidance in Section 2 of experiment EMS, determine the experimental Rydberg constant,…
A: The experimental Rydberg constant is1.09223×107m−1Explanation:Step 1:Wavelength is103nmEmission line…
Q: What is the product of the following reaction? HF C14H20
A: Given reaction refers to the replacement of an aromatic proton with an alkyl group. This is done…
Q: What three alkenes (excluding stereoisomers) can be used to prepare 3- chloro-3-methylhexane by…
A: Stereochemistry is defined as the arrangement of molecule in three dimensional and its impact on…
Q: Consider the compound: H3C CH3 CH3 The common name is: tert-butyl iodide propyl iodide butyl iodide…
A: The given compound is an alkyl halide..Alkyl halide - Organic compounds in which one or more…
Q: Provide reagents and conditions for the following synthetic tranformation. (Please don't provide…
A: In the given reaction the alcohol is converted into carboxylic acid and an extra carbon atom is…
Q: When this redox equation is balanced with the lowest whole number coefficients, on which side does…
A: Oxidation: Loss of electrons with an increase in the oxidation state. Reduction: Gain of electrons…
Q: A B H O Type your answers in all of the blanks and submit X₁ X N- 0 The following compound has both…
A: The objective of the question is to predict the site of reaction on a compound that has both an…
Q: Problem 3. A solution is prepared by dissolving 12.02 g of LiClO to make one liter of solution (Ka…
A: Given:A solution is prepared by dissolving 12.02 g of LiCIO to make one liter of solution.Ka of HClO…
Q: 7. Draw the product of the following reaction. H3C-N H3C CH3
A: Organic reactions can be defined as the reactions in which organic reactants react with each other…
Q: 1 sentence justification of your answer for each item. Without doing any calculations, determine the…
A: Entropy (S) is the degree of randomness.A positive sign of S indicates an increase in entropy and a…
Q: Predict major product of coupling reaction Grubbs Catalyst
A:
Q: Draw the predominant product(s) of the following reactions including stereochemistry when it is…
A: We have to predict the products.
Q: What are the allowed values for each of the four quantum numbers: n, l, m,, Ⓐ n= (1-8); 1= (0 to 8);…
A: To find out all allowed values for each of the quantum numbers.Quantum numbers are defined as those…
Step by step
Solved in 1 steps

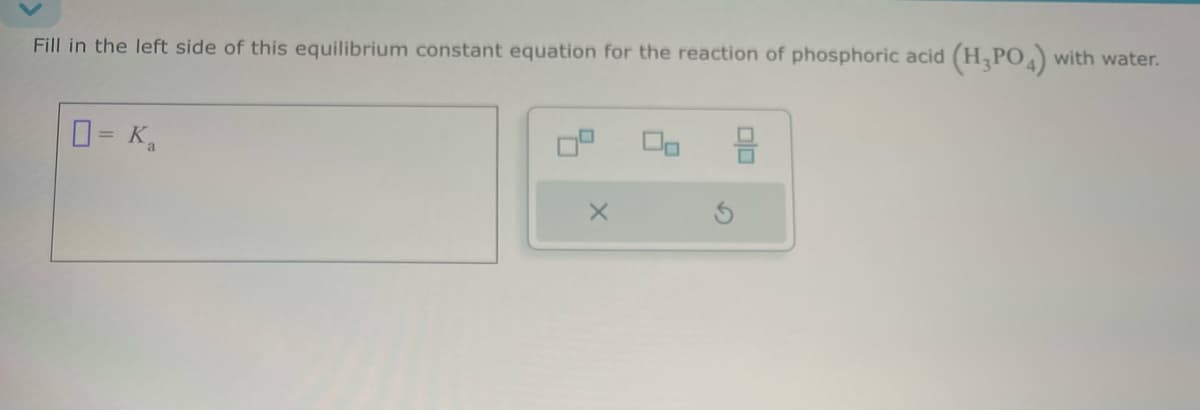
- How many mEq of HCO3 are present in a solution that also contains 75 mEq of Na+, 83 mEq K+, 10 mEq Ca2+, and 153 mEq Cl?Fill in the left side of this equilibrium constant equation for the reaction of acetic acid (HCH3CO2) with water. ____=KaFill in the left side of this equilibrium constant equation for the reaction of phosphoric acid (H3PO4) with water. ____=Ka
- What is the concentration (in molarity) of H+, HCO3−, and CO32–, in a solution that is initially prepared as a mixture of 0.0100 M H2CO3 and 0.000010 M Na2CO3? (Carbonic acid is a weak diprotic acid where Ka1 is 4.20 x 10–5 and Ka2 is 2.00 x 10–6) a) [H+] at equilibrium = ? b) [HCO3-] at equilibrium = ? c) [CO32-] at equilibrium = ?What is the equilibrium constant expression for the following reaction?0, (aq) + Fe2+(aq) + 8H*(aq) -Mn?+(aq) + Fe3+(aq) + 4H20(l)Caproic acid, HC6H11O2, is found in coconut oil and is used in making artificial flavors. A solution is made by dissolving 0.450 mol of caproic acid in enough water to make 2.0 L of solution. The solution has [H3O+] of 1.7 x 10-3 M. What is Ka for caproic acid? Use the ICE table to determine the equilibrium concentrations.
- HNO2 (aq) + NH3 (aq) --> NH4 + (aq) + NO3 - (aq) Kc = 1x 10^6 Nitrous acid reacts with ammonia according to the balanced chemical equation shown above. If 50 mL of 0.20 M HNO2 (aq) and 50 mL of 0.20 M NH3 (aq)are mixed and allowed to reach equilibrium at 25C, what is teh approxiamate [NH3] at equilibrium?Complete the following neutralization reaction. (Predict the products and balance the reaction.) Ca(OH)2 + HClO4 --> ? Group of answer choices Ca(OH)2 + HClO4 --> 2 H2O + CaClO4 Ca(OH)2 + HClO4 --> H2O2 + CaClO4 Ca(OH)2 + HClO4 --> H2O + Ca(ClO4)2 Ca(OH)2 + 2 HClO4 --> 2 H2O + Ca(ClO4)2Show the equilibrium equations for the ionization of CH3COOH and KH2PO4.
- For a given acid, Ka is 7.2 x 10−4. This means that it is: a strong acid B a weak base C a weak acid D a strong base E None of the othersA 0.261 g sample of NaHC2O4 (one acidic proton) required 17.5 mL of sodium hydroxide solution for complete reaction. Write the equation for this reaction and determine the molar concentration of the sodium hydroxide solution.What is the highest concentration of Ag+ that can exist in a solution of 0.15 M NaSO4? [Ag+] = _______ x10 ________ M

