Describes how temperature affects the volume of a gas kept at constant pressure.
Different fundamental gas laws are there that tells the relationship between the pressure, temperature, volume and amount of gas. Ideal gas gives the relation between these variables.
Illustration for the relationship between temperature and volume at constant pressure:
The molecules of gas move straight in container until there comes any partition in between. When molecule encounters any partition, it will bounce and gets any other direction. According to Newton’s third law of motion both molecule and partition will feel some force.
If temperature will be increased gas molecules will tend to move faster. When they move faster they will bounce more forcefully but if volume can be adjusted then volume can be increased and pressure will remain same then.
This is the only relation of Charles Law which tells the relation between temperature and volume at constant pressure.
Relation of Charles Law is given as:
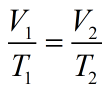
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images









