Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter14: Elimination
Section: Chapter Questions
Problem 36E
Related questions
Question
Transcribed Image Text:H
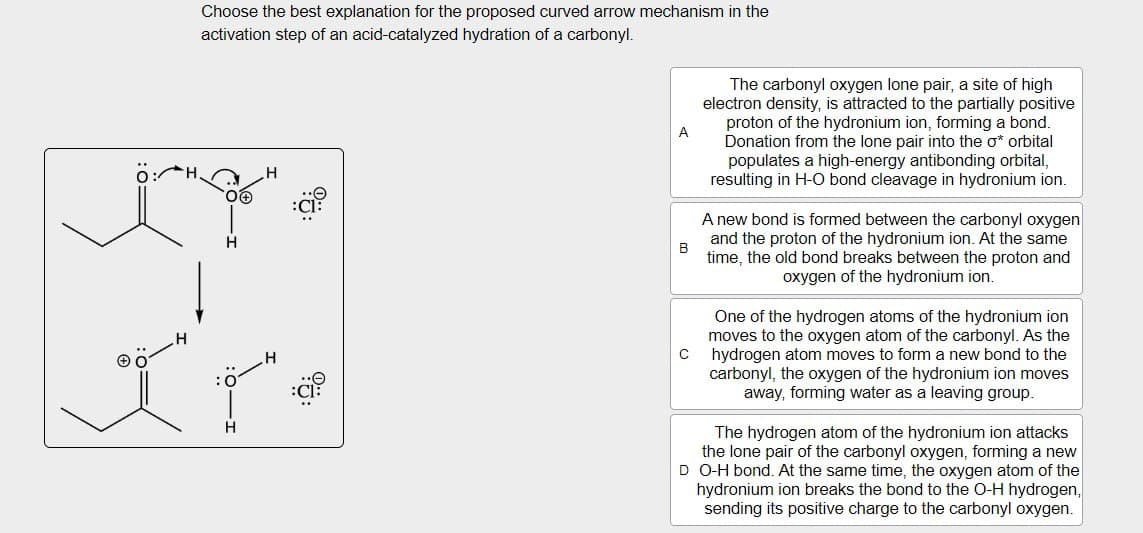
Choose the best explanation for the proposed curved arrow mechanism in the
activation step of an acid-catalyzed hydration of a carbonyl.
H
H
H
9:
:Cl:
A
B
The carbonyl oxygen lone pair, a site of high
electron density, is attracted to the partially positive
proton of the hydronium ion, forming a bond.
Donation from the lone pair into the σ* orbital
populates a high-energy antibonding orbital,
resulting in H-O bond cleavage in hydronium ion.
A new bond is formed between the carbonyl oxygen
and the proton of the hydronium ion. At the same
time, the old bond breaks between the proton and
oxygen of the hydronium ion.
One of the hydrogen atoms of the hydronium ion
moves to the oxygen atom of the carbonyl. As the
C hydrogen atom moves to form a new bond to the
carbonyl, the oxygen of the hydronium ion moves
away, forming water as a leaving group.
The hydrogen atom of the hydronium ion attacks
the lone pair of the carbonyl oxygen, forming a new
D O-H bond. At the same time, the oxygen atom of the
hydronium ion breaks the bond to the O-H hydrogen,
sending its positive charge to the carbonyl oxygen.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
