Attempt 3 NH, is a weak base (K, = 1.8 x 10) and so the salt NH,Cl acts as a weak acid. What is the pH of a solution that is 0.038 M in NH,Cl at 25 °C? pH =
Attempt 3 NH, is a weak base (K, = 1.8 x 10) and so the salt NH,Cl acts as a weak acid. What is the pH of a solution that is 0.038 M in NH,Cl at 25 °C? pH =
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.123E
Related questions
Question

Transcribed Image Text:Attempt 3
NH, is a weak base (K, = 1.8 x 10) and so the salt NH,Cl acts as a weak acid. What is the pH of a solution that is 0.038
M in NH,Cl at 25 °C?
pH =
Expert Solution
Step 1
Dissociation constant of base is given by
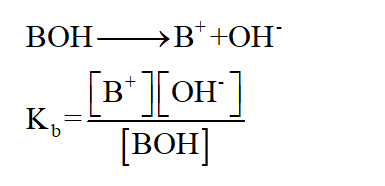
Step 2
The Ka is calculated as
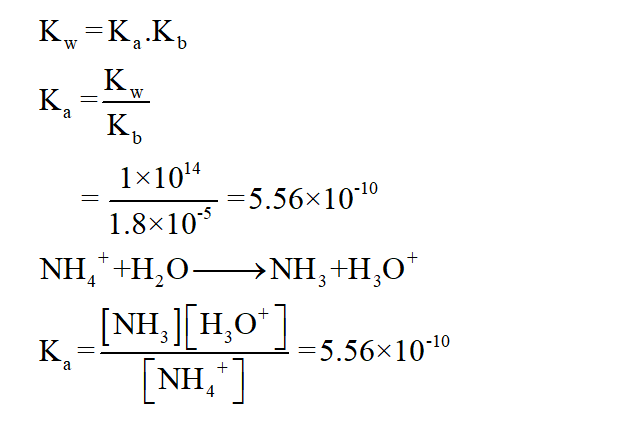
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning