Cis-diols are formed when alkenes are reacted with mCPBA and H3O+. Ketones are always produced from the ozonolysis reactions of alkenes. Select one: O Both statements are true. O Both statements are false. O Only the first statement is true. O Only the second statement is true.
Cis-diols are formed when alkenes are reacted with mCPBA and H3O+. Ketones are always produced from the ozonolysis reactions of alkenes. Select one: O Both statements are true. O Both statements are false. O Only the first statement is true. O Only the second statement is true.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter19: Enolate Anions And Enamines
Section19.9: Crossed Enolate Reactions Using Lda
Problem JQ
Related questions
Question

Transcribed Image Text:Cis-diols are formed when alkenes are reacted with mCPBA and H3O+. Ketones are always produced from the ozonolysis reactions of alkenes.
Select one:
O Both statements are true.
O Both statements are false.
O Only the first statement is true.
O Only the second statement is true.
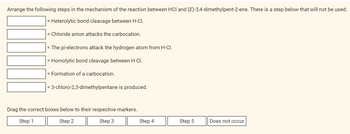
Arrange the following steps in the mechanism of the reaction between HCl and (E)-3,4-dimethylpent-2-ene. There is a step below that will not be used.
= Heterolytic bond cleavage between H-CI.
= Chloride anion attacks the carbocation.
= The pi-electrons attack the hydrogen atom from H-CI.
= Homolytic bond cleavage between H-CI.
= Formation of a carbocation.
3-chloro-2,3-dimethylpentane is produced.
Drag the correct boxes below to their respective markers.
Step 1
Step 2
Step 3
Step 4
Step 5
Does not occur
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Transcribed Image Text:Arrange the following steps in the mechanism of the reaction between HCI and (E)-3,4-dimethylpent-2-ene. There is a step below that will not be used.
= Heterolytic bond cleavage between H-CI.
= Chloride anion attacks the carbocation.
= The pi-electrons attack the hydrogen atom from H-CI.
= Homolytic bond cleavage between H-CI.
= Formation of a carbocation.
= 3-chloro-2,3-dimethylpentane is produced.
Drag the correct boxes below to their respective markers.
Step 1
Step 2
Step 3
Step 4
Step 5
Does not occur
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
