A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1 s Calculate the broadcast wavelength of the radio station 107.9 FM. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HA ?
A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1 s Calculate the broadcast wavelength of the radio station 107.9 FM. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HA ?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter6: The Structure Of Atoms
Section: Chapter Questions
Problem 72IL: The spectrum shown here is for aspirin. The vertical axis is the amount of light absorbed, and the...
Related questions
Question

Transcribed Image Text:Learning Goal:
Several properties are used to define waves. Every wave has a
wavelength, which is the distance from peak to peak or trough to
trough. Wavelength, typically given the symbol A (lowercase Greek
"lambda"), is usually measured in meters. Every wave also has a
frequency, which is the number of wavelengths that pass a certain
point during a given period of time. Frequency, given the symbol v
(lowercase Greek "nu"), is usually measured in inverse seconds
(s). Hertz (Hz), another unit of frequency, is equivalent to inverse
seconds.
The product of wavelength and frequency is the speed in meters
per second (m/s). For light waves, the speed is constant. The
speed of light is symbolized by the letter c and is always equal to
2.998 x 10° m/s in a vacuum, that is,
c = \v = 2.998 x 10° m/s
Another term for "light" is electromagnetic radiation, which
encompasses not only visible light but also gamma rays, X-rays, UV
rays, infrared rays, microwaves, and radio waves. As you could
probably guess, these different kinds of radiation are associated
with different energy regimes. Gamma rays have the greatest
energy, whereas radio waves have the least energy. The energy
(measured in joules) of a photon for a particular kind of light wave is
equal to its frequency times a constant called Planck's constant,
symbolized h :
Ephoton = hv
where
h 6.626 x 10 34 J.s
These two equations can be combined to give an equation that
relates energy to wavelength:
E
hc

Transcribed Image Text:Part A
A radio station's channel, such as 100.7 FM or 92.3 EM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1s1
Calculate the broadcast wavelength of the radio station 107.9 FM.
Express your answer to four significant figures and include the appropriate units.
> View Available Hint(s)
µA
?
Value
Units
Submit
Previous Answers
X Incorrect; Try Again; 2 attempts remaining
Expert Solution
Step 1
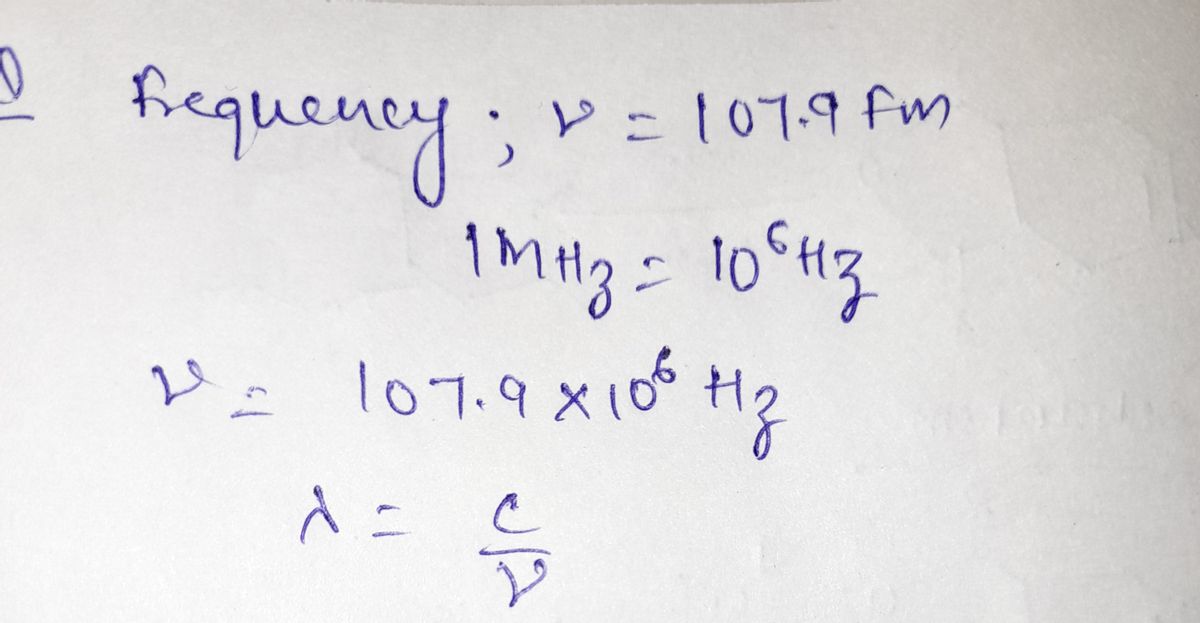
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
