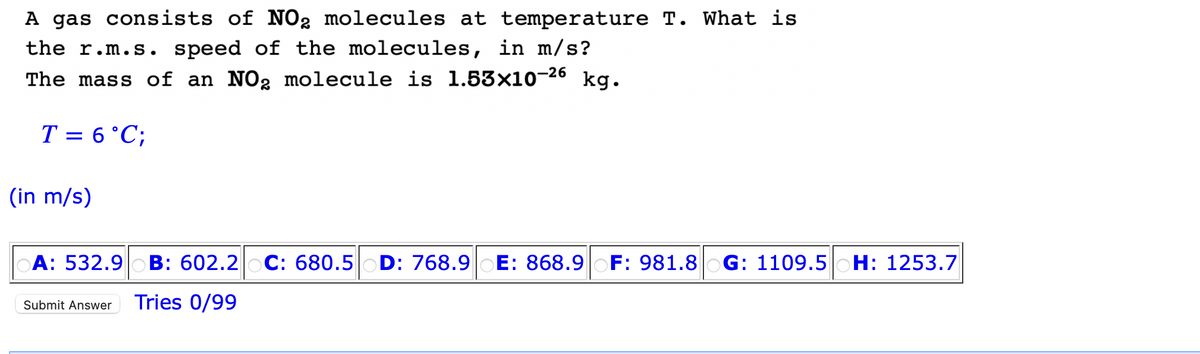
A gas consists of NO₂ molecules at temperature T. What is the r.m.s. speed of the molecules, in m/s? The mass of an NO2 molecule is 1.53x10-26 kg. T = 6 °C; (in m/s) A: 532.9 B: 602.2 C: 680.5 D: 768.9 E: 868.9 F: 981.8 G: 1109.5 H: 1253.7 Submit Answer Tries 0/99
Q: Solar heating of a house is much more efficient if there is a way to store the thermal energy…
A: Here we have to find the mass of the slab. Considering that the slab is 12 m2 in size and 25 cm…
Q: 100 proda (padług und 31 4,0 24,00 PP MAJURIT MIL-2,25 1:22 (O SONHOOD 2020 ARAD 1,5bar MEDEN…
A:
Q: What is the velocity versus time graph given the following data recieved using Arduino Uno.
A: We are given distance time graph. We are given various regions in graph. In each of the region, the…
Q: A 5.0-cm tall object stands in front of a converging lens. It is desired that the virtual image is…
A:
Q: If we place a particle with a charge of 1.4 x 10° C at a position where the electric field is 8.5 x…
A: Given: In this question, the given details are, The given charge value is, q=1.4×10-9C, And the…
Q: Is the number of molecules in one mole of N₂ greater than, less than, or equal to the number of…
A: We must establish if there are more, less, or the same number of molecules in one mole of N2 as…
Q: A body weight 4.5 kg wt on the surface of earth. How much will it weight on the surface of the…
A:
Q: 5. What is the mass of a black hole formed at the beginning of the universe that would explode by…
A:
Q: The path difference between two identical waves arriving at a point is 100.5 A. Is the point bright…
A: We need to compute-(i) Whether the point is bright or dark=?(ii) Wavelength of light λ=?The data…
Q: : Biprism experiment is conducted with a wavelength of 5000 Å. The distance between the virtual…
A: We need to compute-Distance between consecutive bright and dark bands=?The data given as-Wavelength…
Q: A 10 nF air capacitor is connected to a power supply, the energy stored in the capacitor is 125 x…
A: Given data, Capacitance of empty capacitor C0=10 nF. Energy stored in empty capacitor U0=12.5×10-5…
Q: Four wave functions are given below. Rang them in order of the magnitude of the wave periods, from…
A:
Q: QUESTION 2 Along wire cares a current out of the page. Which of the following best represents the…
A: Right hand thumb rule: Thumber of your right hand placed in the direction of current. The curl of…
Q: When two parallel plates connected to a 40-V battery, the electric field between the plates is 2200…
A: Given: In this question, the given values are, Voltage is, V=40Volt, And the electric field is,…
Q: An airplane flying at an altitude of 12,000m at 80 m/s. What is the difference of total pressure and…
A: Given data, Height is given h = 12000 m. Velocity v = 80 m/s.
Q: ..A Rowland ring of means radius 16 cm Mas 1000 turns of wire closely wound on ferromagnetic core of…
A: We need to compute-Magnetic induction (B)=?The data given as-radius (r)=16 cm=0.16 mNo. of turns…
Q: A bay magnet is oscillating in a uniform magnetic field of induction 0.4×10°³7. When the frequency…
A: We need to compute-Increase in magnetic induction (B2)=?The data given as-Magnetic induction…
Q: The presence of dark energy affects to the way the Universe is expanding. Select one: True False
A: Dark energy is a hypothetical form of energy which acts opposite to gravity. It occupies the…
Q: Electromagnetic Wave We have a plane electromagnetic wave traveling in the +z direction. As you may…
A:
Q: Cool Medicine in situations in which the brain is deprived of oxygen, such as in a heart attack or…
A: Information provided: The body's core temperature is 37 °C.Human body's interior core temperature is…
Q: We studied how a moving charge would react in a magnetic field, which extends aturally to current…
A: We have to find current. We have to assume all unknowns as said in question. We first find…
Q: A golf ball is driven at an angle of 45.0 O against the horizontal at an initial velocity of 40.0…
A: Disclaimer: “Since you have asked posted a question with multiple sub-parts, we will solve the first…
Q: A camera has a converging lens with a focal length of 56 mm. If the f-number is 2.8 what is the…
A: Given; Focal length,F=56 mmf-number,f=2.8 The ratio of the focal length of the system to the…
Q: B. A car started from rest and move with constant acceleration. At one time, it was travelling…
A:
Q: how that a sphere of radius R that is rolling without slipping has angular momentum and momentum in…
A: Moment of inertia times angular velocity defines angular momentum as the property of any rotating…
Q: Write down jones vector that is polarized at 30° with the x-axis. What would be the direction of…
A: Jones vector is the way to express the equation of wave in matrix forms. It is a simple way and…
Q: The hot-air balloon contains air having a temperature of 180°F while the surrounding air has a…
A: Given data, Temperature of hot air is 180°F. Temperature of surrounding air is 60°F. Density of air…
Q: 22. (a) Solve the classical wave equation governing the vibrations of a stretched string, for a…
A:
Q: The average speed of air molecules in your room is on the order of the speed of sound. What is their…
A: We must determine the average air molecule speed in a space and demonstrate that it is comparable to…
Q: A torque of magnitude 2000 Nm acting body produces an angular acceleration of 2 rad/s². Calculate…
A: We know that a body under a 2000 Nm torque will accelerate angularly at a rate of 2 rad/s2.
Q: A 5.9-kg block of ice at -1.5 °C slides on a horizontal surface with a coefficient of kinetic…
A: We must determine the value of m in this problem. The ice block's mass is 5.9 kg, its beginning…
Q: A uniform solid cylinder is placed with its axis horizontal on a plane, whose inclination to the…
A:
Q: Moment of inertia of a solid sphere of 7 mass (M) and radius (R) about it tangent is 5 MR². Find its…
A: We are aware that a solid sphere with mass M and radius R has a moment of inertia about its tangent…
Q: Ex./51: M.I. of a uniform disc about an axis passing through its centre and perpendicular to its…
A: We need to compute-M.I. of uniform disc about an axis passing through any diameterThe data given…
Q: A slone weighing 1kg is whirled in a vertical circle at end of a rope of length 0.5m. Find lension…
A: We need to compute-(i) Tension at lowest position (TL)=?(ii) Tension at midpoint (TM)=?The data…
Q: What is the surface current density flowing on the outer surface of the ferromagnetic material
A: Surface current density flowing on the outer surface of the ferromagnetic material. Given, magnetic…
Q: An infinite rectangular slab of thickness 2d is situated parallel to the x-y plane, with its center…
A: Magnetic fields are regions in which magnetism acts around magnetic materials or moving electric…
Q: Prove that the momentum operator is interchangeable with the total energy operator.
A: We know that moment operator and total energy operator is interchangeable. We can prove it as…
Q: A 10.0-V battery, a 5.00-Q resistor, and a 10.0-H inductor are connected in series. After the…
A: Inductors are passive devices that are capeable of storing energy in them as magnetic fields. When a…
Q: A rock is dropped from high above the surface of the Earth. The initial speed is 0, and the initial…
A:
Q: An infinite rectangular slab of thickness 2d is situated parallel to the x-y plane, with its center…
A: Given Data: Let us consider the given infinite rectangular slab of thickness 2d is situated parallel…
Q: 14. Which theory describes the behavior of systems with accelerating frames of reference or under…
A: "The student has specifically asked to answer question 16" The event horizon for the black hole is…
Q: If the current density in a cop b. C. 0.138 V/m Not enough information d. 7.25 V/m 4.64 x 10¹4 V/m
A: Given data, Conductivity of copper σ=5.8×107 S/m. Current density J=8×106 A/m2.
Q: Ex. 63: A ballet dancer spins about vertical axis at 90 r.p.m. with arms outstretched. With the arms…
A: We have to compute-New frequenncy of revolution (n2)=?The data given as-Initial frequency (n1)=90…
Q: Two long current carrying wires, I and I2, are separated by 2 mm and both carry a current of 8 A.…
A:
Q: Problem 1.1 What is the relationship between e, and e, and the usual Cartesian vectors i and j? By…
A:
Q: 4) A uniform disk and a hoop each have the same mass and radius. They each spin with the Which has…
A: We will answer this question using formula for rotational kinetic energy. The details are given…
Q: how did you do the calculation to find the pointing vector for the maximum points because I am…
A: Yes ! You are absolutely correct. There's a calculation error in previous solutions. Please have a…
Q: You are working on the plans for an expedition to colonize Mars. In an effort to provide a homey…
A: (a) According to the question, the clock pendula has a small attached at the bottom of the light…
Q: Ex. 50: The work function of a metal is 4.3 eV. Find the K.E. and velocity of photoelectrons emitted…
A: We need to compute-(1) KE=?(2) Velocity (V)=?The data given as-Work function (W0)=4.3 eVWavelength…
Step by step
Solved in 2 steps with 2 images
