* 97% 7:58 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 Rate law of a reaction is experimentally determined as Rate = k [A]^2 [B]^0 [C] The unit of k is sec/M 1/M^3-sec 1/M^2-secc. 1/M^4-sec M^3/sec Submit
* 97% 7:58 PM Wed Feb 12 х Unanswered •1 attempt left • Due on Feb 13 Rate law of a reaction is experimentally determined as Rate = k [A]^2 [B]^0 [C] The unit of k is sec/M 1/M^3-sec 1/M^2-secc. 1/M^4-sec M^3/sec Submit
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.20P
Related questions
Question
![* 97%
7:58 PM Wed Feb 12
х
Unanswered •1 attempt left • Due on Feb 13
Rate law of a reaction is experimentally determined as
Rate = k [A]^2 [B]^0 [C]
The unit of k is
sec/M
1/M^3-sec
1/M^2-secc.
1/M^4-sec
M^3/sec
Submit](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fcf9e4020-8e90-4f66-8899-b332a9e4e6d5%2Ff71143e4-2c1b-4d58-9a98-3f8a0e001898%2Fybtz12i.png&w=3840&q=75)
Transcribed Image Text:* 97%
7:58 PM Wed Feb 12
х
Unanswered •1 attempt left • Due on Feb 13
Rate law of a reaction is experimentally determined as
Rate = k [A]^2 [B]^0 [C]
The unit of k is
sec/M
1/M^3-sec
1/M^2-secc.
1/M^4-sec
M^3/sec
Submit
Expert Solution
Step 1
The unit of rate constant, k of any order can be determined using the formula :
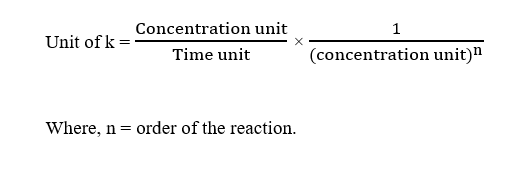
Step 2
Given,
Rate = k [A]2 [B]0 [C]
The order of the reaction = 2 + 0 + 1 = 3
Here, concentration unit = M
Time unit = sec
Therefore, the unit of k can be calculated using the above formula as:
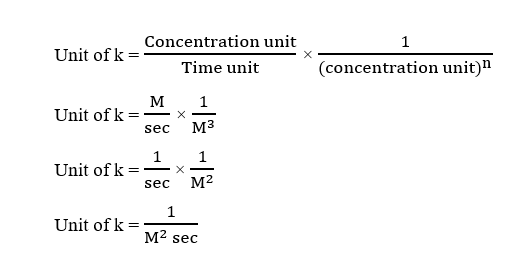
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning