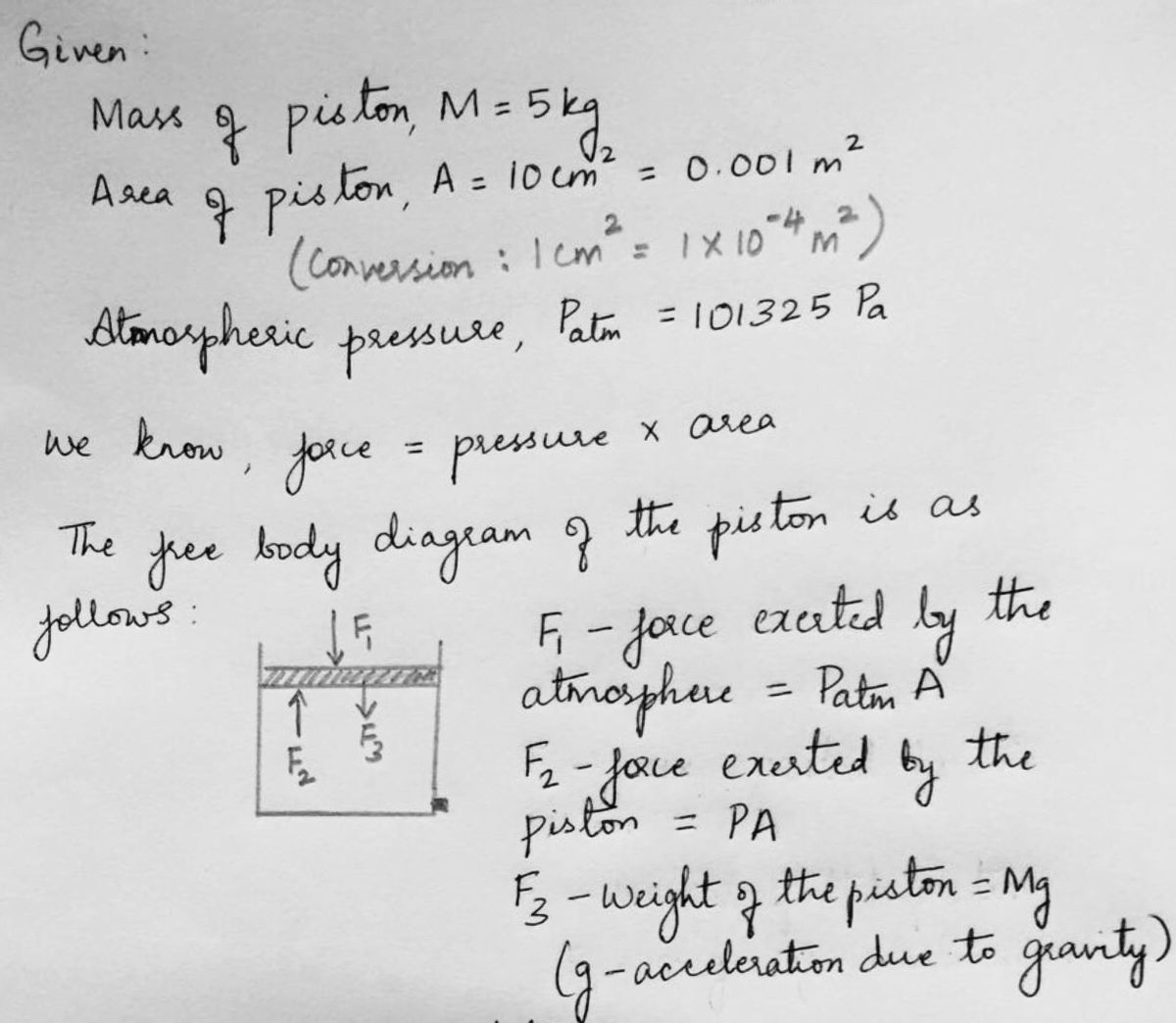
A cylinder filled with ideal gas has a piston of mass M=5kg and area A=10 cm?. The cylinder has a valve located at the bottom. The valve was initially opened and some of the gas escapes slowly. The valve is then closed, after which the piston is observed to be at a lower position. Assume that the system is in thermal equilibrium with the surroundings and the piston is allowed to move freely during the whole process. 1. What was the initial absolute pressure of the ideal gas before the valve was opened?
A cylinder filled with ideal gas has a piston of mass M=5kg and area A=10 cm?. The cylinder has a valve located at the bottom. The valve was initially opened and some of the gas escapes slowly. The valve is then closed, after which the piston is observed to be at a lower position. Assume that the system is in thermal equilibrium with the surroundings and the piston is allowed to move freely during the whole process. 1. What was the initial absolute pressure of the ideal gas before the valve was opened?
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter19: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 40AP: One mole of an ideal gas is contained in a cylinder with a movable piston. The initial pressure,...
Related questions
Question
100%

Transcribed Image Text:A cylinder filled with ideal gas has a piston of mass M=5kg and area
A=10 cm?. The cylinder has a valve located at the bottom. The valve was
initially opened and some of the gas escapes slowly. The valve is then
closed, after which the piston is observed to be at a lower position.
Assume that the system is in thermal equilibrium with the surroundings
and the piston is allowed to move freely during the whole process.
1. What was the initial absolute pressure of the ideal gas before the
valve was opened?
Is the final pressure of the gas in the cylinder greater than, less than, or equal to the initial
pressure? Explain.
Explain how your answer is consistent with the forces acting on the piston in the initial and
final states.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning